Gypsum is one of the most common minerals in the world. Sometimes you can find its varieties - silky twine, Maryino glass or Ural selenite. Gypsum stone has unique properties and influence on different zodiac signs. The mineral gipsos is translated from Greek as chalk or gypsum. In nature, it is found pink, white or cream in color.
What is gypsum
For most people, plaster is a hard, opaque grayish substance that is placed on a broken arm or leg in the hospital.
However, the description of the natural mineral is richer:
- It can be half or completely transparent, translucent, even luminous.
- Gloss – pearlescent, glassy, silky, matte.
- More often it is presented as a tabular agglomerate or crystals - columns, prisms, needles.
The mineral cannot be dissolved by most acids, but water has no problem.
This characteristic of gypsum is unique: solubility with water is maximum at 37.8°, after which it tends to zero.
Story
The mineral received its name in 315 BC, it was discovered by Tsophrastus. In ancient times, gypsum was used not only for agricultural purposes, embedding it into the soil and increasing crop yields, but during construction, cutting blocks from it. For example, several city walls in Syria were built from plaster. Even today you can see the remains of the walls, shining white in the sun.
Legend
The mineral traces its history back to Ancient Egypt, where sages invented unique recipes. Among the various legends, it is worth highlighting the story of how gypsum mortar was used during the construction of Khafre. It was the second largest Egyptian pyramid. Thanks to the sages, a unique composition was made, the recipe of which has not been preserved to this day. But even today the integrity of the pyramids, which have stood for more than one millennium, is visible.
Physico-chemical characteristics
According to chemical nomenclature, the mineral gypsum is aqueous calcium sulfate. The international classification defines the mineral class as sulfates.
Its composition is complex, the formula is multi-component.
| Formula | CaSO4 2H2O |
| Color | White, shades of gray and red |
| Stroke color | White |
| Shine | Glass to pearlescent |
| Hardness | 1,5—2,0 |
| Cleavage | Very perfect |
| Kink | Uneven; flexible but not elastic |
| Density | 2.2—2.4 g/cm³ |
| singonia | Monoclinic |
| Refractive index | 1,52 |
Translation into other languages
|
|
Place of Birth
Sedimentary origin ensured the widespread occurrence of the rock on the planet:
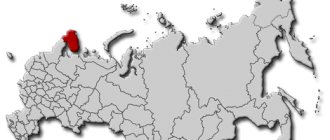
- Russian deposits are concentrated in the North Caucasus, in the vicinity of the Urals, Krasnodar Territory, Tatarstan, and Dagestan.
- The largest suppliers of raw materials to the world market are the USA, Canada, Spain, Iran, and Turkey.
Some mines are unique. For example, in Oklahoma. This US state has an array of natural gypsum formations - the Alabaster Caves park with raw materials of white, pink and rare black color. However, they extract it there in crumbs.
Origin and distribution in nature
Gypsum is the most common sulfuric acid salt in nature.
Sometimes entire gypsum caves are formed. The length of the longest of them is more than 230 km. Gypsum is mainly of sedimentary origin - it is formed by chemical precipitation during the drying of salt water bodies rich in sulfates. The largest layers occur off the coast of inland seas, in lagoons, in shallow sea waters, and at the mouths of ancient rivers. Gypsum can also be formed during the weathering of sulfur deposits, but such deposits contain a large amount of impurities. Gypsum of metasomatic origin is also found in nature. In this case, the mineral is formed as a result of the replacement of rocks, which is accompanied by a change in the chemical and mineral composition under the influence of external factors.
Varieties of mineral
Depending on the structure, density, and other characteristics, several types of gypsum are distinguished:
- Alabaster. The whitest mineral of high purity. Among the Greeks, the term αλαβαστρος meant “white.” Formed when gypsum is heated to 142°C.
- Selenite. A colorless variety of fibrous structure with a silky sheen. Found a century and a half ago in the Urals. Named due to the radiance that seems to emanate from within the stone. Based on this characteristic, it can be easily distinguished from other types of gypsum.
In Russia it is known as “Maryino glass”. The history of the name is connected with the tradition of covering the faces of saints, especially the Mother of God (Virgin Mary), with transparent selenite plates.
- Desert Rose. Plaster plates of pastel shades, assembled in the shape of a rosebud. Found in the deserts of Africa.
- Crystal. Not a particularly durable mineral with grayish tints. Goes for souvenirs.
- Anhydride. Dehydrated gypsum in the form of crystals (sometimes very large). Looks like marble. It is easy to distinguish the origin by placing the sample in a humid microclimate. The plaster will gradually swell and become deformed.
Mineral Anhydride
There is a classification of the mineral according to the speed of setting (fast-, medium-, slow-hardening gypsum).
Origin
A widespread mineral, it is formed in natural conditions in various ways. The origin is sedimentary (typical marine chemogenic sediment), low-temperature hydrothermal, found in karst caves and solfataras. Precipitates from sulfate-rich aqueous solutions during the drying out of sea lagoons and salt lakes. Forms layers, layers and lenses among sedimentary rocks, often in association with anhydrite, halite, celestine, native sulfur, sometimes with bitumen and oil. It is deposited in significant quantities by sedimentation in lake and sea salt-bearing dying pools. In this case, gypsum, along with NaCl, can be released only in the initial stages of evaporation, when the concentration of other dissolved salts is not yet high. When a certain concentration of salts, in particular NaCl and especially MgCl2, is reached, anhydrite will crystallize instead of gypsum and then other, more soluble salts, i.e. The gypsum in these basins must belong to earlier chemical sediments. Indeed, in many salt deposits, layers of gypsum (as well as anhydrite), interbedded with layers of rock salt, are located in the lower parts of the deposits and in some cases are underlain only by chemically precipitated limestones.
Significant masses of gypsum in sedimentary rocks are formed primarily as a result of the hydration of anhydrite, which in turn was deposited during the evaporation of sea water; Often, when it evaporates, gypsum is directly deposited. Gypsum arises as a result of the hydration of anhydrite in sediments under the influence of surface waters under conditions of low external pressure (on average to a depth of 100-150 m) according to the reaction: CaSO4 + 2H2O = CaSO4 × 2H2O. In this case, a strong increase in volume occurs (up to 30%) and, in connection with this, numerous and complex local disturbances occur in the conditions of occurrence of gypsum-bearing strata. In this way, most of the large deposits of gypsum on the globe arose. In the voids among solid gypsum masses, nests of large, often transparent crystals are sometimes found.
Can serve as cement in sedimentary rocks. Vein gypsum is usually a product of the reaction of sulfate solutions (formed by the oxidation of sulfide ores) with carbonate rocks. It is formed in sedimentary rocks during the weathering of sulfides, under the influence of sulfuric acid formed during the decomposition of pyrite into marls and calcareous clays. In semi-desert and desert areas, gypsum is very often found in the form of veins and nodules in the weathering crust of rocks of various compositions. In the soils of the arid zone, new formations of secondary redeposited gypsum are formed: single crystals, twins (“swallowtails”), druses, “gypsum roses,” etc.
Gypsum is quite soluble in water (up to 2.2 g/l), and with increasing temperature its solubility first increases, and above 24 ° C it decreases. Due to this, gypsum, when deposited from sea water, is separated from halite and forms independent layers. In semi-deserts and deserts, with their dry air, sharp daily temperature changes, saline and gypsum-filled soils, in the morning, as the temperature rises, gypsum begins to dissolve and, rising in the solution by capillary forces, is deposited on the surface as water evaporates. In the evening, as the temperature drops, crystallization stops, but due to lack of moisture, the crystals do not dissolve - in areas with such conditions, gypsum crystals are found in especially large quantities.
Where is it used?
The scope of application of gypsum is limitless. Each uses the right type of raw material.
Application areas
This is an inexpensive, practical material for cement, slabs, blocks, cornices. Suitable for interior or exterior.
Alabaster is important as a raw material in the production of special grades of paper, enamels, paints, glazes, and medical compositions.
The substandard material is ground and turned into a soil desalinizer.
Aesthetics
Sculptors cannot work without plaster blanks.
Master stone carvers turn stone into small plastic pieces, vases, and boxes.
Colorless transparent selenites are especially in demand. Stone cutters transform mysteriously shimmering pebbles into small plastic pieces, an esoteric assortment: pyramids, balls, pendulums.
Selenite bracelet
Jewelers create cabochons.
However, the fragility of the mineral limits the range. These are mainly pendants, pendants, brooches - things that do not risk quickly wearing out or crumbling.
Collecting
You can collect the “gypsum section” of a mineralogical collection for years, the manifestations and forms of the mineral are so diverse.
Connoisseurs are especially interested in “desert rose”, “Maryino glass”, black and pink stones from America, “dovetail”, samples with the cat’s eye effect.
Maryino glass
Material storage
The only reliable way to properly store dry gypsum material is to use glass jars with a sealed lid. Dry calcined gypsum can be used to drain containers or floors, but to restore the original qualities of the material, it is necessary to deoxidize it with an aqueous solution of sulfuric acid, remove the water by calcination and re-grind it into dust to a grain size of 0.01-0.003 mm. Industrial polyethylene packaging provides reliable storage of the dry mixture only for the first two months. Dry plasters based on gypsum material in paper bags after opening must be used within 3 days.
How to care
Plaster is strong, but vulnerable, so you need to care for it carefully:
- Avoid falls, impacts, and mechanical impact.
- Protect stones from the harsh sun (especially alabaster, which quickly turns yellow and fades).
- Do not place products in rooms with consistently high humidity (bathtub, swimming pool, open veranda, greenhouse).
A humid microclimate is detrimental to gypsum: the mineral becomes saturated with water, losing its shape and decorativeness.
Contaminants are removed from the stone with a dry or slightly damp cloth.
It is useful to recharge the selenite variety of the mineral with the light of the Moon by placing it on the windowsill at night.
Advantages of gypsum stone
Decorative gypsum stone is used for interior decoration of premises. Its advantages when mounted on a wall are ease and convenience in construction work. Products made from dihydrous gypsum are unpretentious in installation and they do not weigh down the walls, unlike natural stones, which are difficult to lay evenly due to their varying thickness, heaviness and uneven edges. Natural stone tiles are very difficult to install and are not recommended for thin walls. Gypsum masonry does not create such a problem; it allows you to quickly and efficiently formalize a design idea, leaving the dimensions of the room the same, and the appearance is transformed in a refined modern way.
Advantages of gypsum stone
Along with its enormous advantages, gypsum also has some disadvantages. So, if you decide to buy decorative gypsum stone, then you need to be very careful when transporting it, because it easily breaks when dropped. Also, when transporting stone over significant distances, it is recommended to purchase tiles with a more pronounced thickness.
When choosing a stone in construction stores, pay attention to whether it is painted only on the outside or inside. This will be clearly visible on the saw cut. If the stone is painted only on the outside, then with small chips and scratches it will be noticeable. You can also purchase unpainted gypsum tiles and paint them the color you want after installation. It is recommended to do this using acrylic paint and cover the top with acrylic varnish.
If you want to make decorative stone yourself, it is better to purchase iron oxide dye in advance so that the entire gypsum solution is colored before pouring into molds. This way you can make tiles of different shades, and by additionally painting the mold before pouring it, you will achieve the effect of streaks like natural stone.
And of course, your decorative tiles will be no different from the real thing, even to an experienced eye. After all, in order to compare it with a natural sample, it must be removed from the wall and broken. Of course, this won't happen. Therefore, no one will be able to understand how your interior is decorated. Modern forms for artificial pouring perfectly replicate all the bends, cracks and roughness of natural rocks, and gypsum penetrates deeply into the smallest pores and repeats the pattern in great detail. Gypsum stone will serve you for many years, because its temporary life is from 15-20 years. It is deformed only during mechanical actions. If you are not going to drop furniture on your wall, then your tiles are not in danger.
Therapeutic effect
The healing properties of gypsum are recognized by lithotherapists and official medicine.
Medical science uses the mineral in the following areas:
- Treatment of broken bones or sprained ligaments.
- Regulation of sweating.
- Cleansing the skin and body in general. There is no mysticism - this is the merit of calcium and sulfur in the composition of the mineral. They are the ones who draw out toxins, impurities, and clean pores.
Lithotherapists recommend the mineral to patients with spinal tuberculosis and osteomyelitis. The pebble is applied to any sore spot.
For a physically healthy person, the mineral is suitable as a sedative. Contemplating a ball or stone for a few minutes a day calms you down, helps you concentrate, and overcome apathy, depression, and anger.
Origin and location
Gypsum is formed in natural conditions in various ways.
- It is deposited in significant quantities by sedimentation in lacustrine marine salt-bearing dying pools. In this case, gypsum, along with NaCl, can be released only in the initial stages of evaporation, when the concentration of other dissolved salts is still low. When a certain concentration of salts, in particular NaCl and especially MgCl2, is reached, anhydrite will crystallize instead of gypsum, then other, more soluble salts will crystallize. Consequently, the gypsum in these basins must belong to earlier chemical sediments. Indeed, in many salt deposits, layers of gypsum (as well as anhydrite), interbedded with layers of rock salt, are located in the lower parts of the deposits and in some cases are underlain only by chemically precipitated limestones.
- Very significant masses of gypsum arise as a result of the hydration of anhydrite in sediments under the influence of surface waters under conditions of low external pressure (on average to a depth of 100–150 m) according to the reaction: CaSO4 + 2H2O = CaSO4. 2H2O
In this case, a strong increase in volume occurs (up to 30%) and, in connection with this, numerous and complex local disturbances occur in the conditions of occurrence of gypsum-bearing strata. In this way, most of the large deposits of gypsum on the globe arose. In the voids among solid gypsum masses, nests of coarse, often transparent crystals (“spary gypsum”) are sometimes found.
- In semi-desert and desert areas, gypsum is very often found in the form of veins and nodules in the weathering crust of rocks of various compositions. It is also often formed on limestones under the influence of water enriched with sulfuric acid or dissolved sulfates. Finally, it is found in oxidation zones of sulfide deposits, but not in such large quantities as might be expected. The fact is that in the overwhelming majority of cases, sulfide ores contain pyrite or pyrrhotite in varying quantities, the oxidation of which (especially the first) significantly increases the content of sulfuric acid in surface waters. Water acidified with sulfuric acid significantly increases the solubility of gypsum. Therefore, in a number of deposits, gypsum is more common in the upper parts of primary ore zones, where it occurs in cracks along with other sulfates.
- Relatively rarely, gypsum is observed as a typical hydrothermal mineral in sulfide deposits formed under conditions of low pressure and temperature. In these deposits it is sometimes observed in the form of large crystals in voids and contains inclusions of chalcopyrite, pyrite, sphalerite and other minerals. Pseudomorphoses of calcite, aragonite, malachite, quartz and other minerals from gypsum have been repeatedly established, as well as pseudomorphoses of gypsum from other minerals.
A rare example of endogenous (hydrothermal) gypsum is the transparent single-crystal masses grown on top of brushes of zeolite crystals in the cavities of the gabbroids of the Talnakh deposit (Norilsk group, Krasnoyarsk region).
Typical marine chemical sediment. By origin and occurrence in nature it is closely related to anhydrite. May form during dehydration of anhydrite. It is also formed in the weathering zone of sulfides and native sulfur (the so-called gypsum hats). Like anhydrite, gypsum can sometimes be of hydrothermal origin, occurring in products of fumarolic activity.
Magic properties
The magical effect of gypsum is ambiguous:
- The stone calms the boiling of passions. A figurine or mineralogical specimen is suitable for hot-tempered, nervous people as a sedative.
The magic of gypsum attracts prosperity, love, money to the owner.
- The crystalline variety of the mineral neutralizes the negative effects of gadgets. It is recommended to place a product made from it near the computer screen.
- The mineral does not suppress a person’s will, but it is not needed by suggestible, insecure people: under its influence, these qualities will intensify.
- The stone is capable of destroying the Napoleonic plans of proud, vain, stubborn, aggressive people.
It is advised to place it in the bedroom so that the marriage remains strong.
Interior decoration with artificial stone
Do-it-yourself decorative stone for interior wall decoration is attached indoors immediately after drying or later. If you have free time, you can make ten to fifteen square meters of stone per day. The price for the materials used will be several times lower than store offers.
For installation, you need to purchase a special adhesive composition at any hardware store. Since the compositions are different, you need to use one that is compatible with gypsum products. There are several such adhesive compositions, so it will not be difficult to choose one of them. The glue batch should be prepared in accordance with the instructions indicated on the packaging. After this, the wall is leveled and the top layer of plaster is necessarily impregnated with a primer for good adhesion to the tile. There is no need to level it very much, because the stone itself will smooth out errors, but it is worth remembering that the amount of adhesive material consumed depends on the degree of evenness of the wall, which is usually applied no thicker than 5 mm with a special slotted spatula.
Installation of decorative stone on the wall
Artificial stone for interior decoration begins to be laid on the wall, starting from the corners. There are special corner shapes, but you can do without them. Of course, then cutting of the stone is inevitable. The tiles are laid from the bottom up to avoid slipping, but some craftsmen lay it the other way around to avoid getting glue on the work already done. If the tiles are not laid from the floor itself, then a metal profile is placed under it for support. When laying out an image, it is recommended to start installation with it. After installing and drying the tiles, after three days it is advisable to start grouting the joints. You can buy the filling material or make it yourself. It is convenient to do this with a large syringe.
After all elements of the pattern have dried, the artificial decorative stone for interior decoration is painted and covered with various coatings. It is usually coated with a primer, followed by dye and acrylic varnish to complete the desired look. The varnish will also protect the plaster from dust and moisture.