Today, ultraviolet light is used to solve a wide variety of problems, including the search for minerals. When exposed to ultraviolet light, various minerals begin to fluoresce, that is, they glow in different colors and shades. Thanks to this, in the dark it is very easy to distinguish a noble stone from ordinary gray stones. This effect is actively used not only directly to search for minerals and ores, but also to study them: determining the purity of stones, as well as the presence of various impurities in their composition.
Story
Vases, bowls, and other dishes that glow under the rays of the sun were made back in Ancient Greece. There these products were available only to the nobility.
Medieval alchemists, conducting experiments on fluorite crystals, noticed that when heated they began to glow in the dark. Often such tests ended in an explosion, and the experimenters were poisoned by poisonous gases. For this, the mineral was nicknamed the devil's stone.
Fluorite has other names:
- ore flower;
- South African emerald;
- fluorspar;
- Murin.
In 1529, the founder of mineralogy, the German scientist Gregory Agricola, came up with the name “fluores” for the stone because of its fusibility (translated from Latin as “flow”, “fluid”). Then the name “fluorite” was assigned to the mineral.
From the name of the stone comes the word “fluorescence,” which refers to the glowing effect under ultraviolet rays or other light sources.
Fluorescent minerals
Oct 19, 2017
What is a fluorescent mineral?
All minerals have the ability to reflect light. This is what makes them visible to the human eye. Some minerals have an interesting physical property known as "fluorescence." These substances have the ability to temporarily absorb small amounts of light and instantly release it at a different wavelength. This change in wavelength causes a temporary change in the color of the mineral.
The color changes of fluorescent minerals are most impressive when they are illuminated in the dark with ultraviolet light (invisible to humans).
More about fluorescence
Fluorescence in minerals occurs when a sample is illuminated with a specific wavelength of light. Ultraviolet (UV) light, X-rays, and cathode rays are typical types of light that cause fluorescence. These types of light have the ability to excite sensitive electrons in the atomic structure of a mineral. The excited electrons temporarily jump to a higher orbit within the mineral's atomic structure. When these electrons return to their original orbit, a small amount of energy is released in the form of light. This process of releasing light is known as fluorescence. The wavelength of light released from a fluorescent mineral is often noticeably different from the wavelength of incident light. This leads to a noticeable change in the color of the mineral. The glow continues as long as the mineral is illuminated by light of the appropriate wavelength.
How many minerals glow under UV light?
Most minerals do not have noticeable fluorescence. Only about 15% of minerals have a glow that people can see, and other examples of these minerals will not glow. Fluorescence usually occurs when certain impurities known as "activators" are present in the mineral. These activators are usually metal cations such as tungsten, molybdenum, lead, boron, titanium, manganese, uranium and chromium. Rare earth elements such as europium, terbium, dysprosium and yttrium are known to contribute to the fluorescence phenomenon. The glow of minerals can also be caused by structural defects in the crystals or organic impurities.
Some impurities quench fluorescence. If iron or copper is present as impurities, they can reduce or eliminate fluorescence. In addition, if the mineral activator is present in large quantities, it may reduce the fluorescence effect.
Most minerals glow in one color. Other minerals have multiple fluorescent colors. Calcite is known to glow in red, blue, white, pink, green and orange. Some minerals exhibit multiple colors of fluorescence in a single sample. These may be banded minerals that exhibit several stages of growth from initial solutions with varying compositions. Many minerals fluoresce one color under short-wave UV light and a different color under long-wave UV light.
Fluorite: the original "fluorescent mineral"
One of the first people to observe fluorescence in minerals was George Gabriel Stokes in 1852. He noted fluorite's ability to produce a blue glow when illuminated with invisible light "beyond the violet end of the spectrum." He called this phenomenon "fluorescence." This definition is widely accepted in the fields of mineralogy, gemology, biology, optics, commercial lighting and many other fields.
Most fluorite samples have fairly strong fluorescence. An observer can take them outside, hold them in sunlight, then move them into the shade and see the color of the mineral change. Only a few minerals have this level of fluorescence. Fluorite typically glows blue-violet under short and long wavelength light. Some samples are known to accumulate cream or white color, but many do not fluoresce. It is assumed that the glow in fluorite is due to the presence of yttrium, europium, samarium or organic material as activators.
Fluorescent geodes?
You may be surprised to learn that geodes (geological formations) have been found with fluorescent minerals inside. Some of the geodes discovered near the community of Dugway, Utah, are filled with chalcedony, which produces a lime-green fluorescence caused by the presence of traces of uranium.
These geodes are amazing for another reason. They formed several million years ago in archaeolite gas pockets. Then, about 20,000 years ago, they were destroyed by wave action along the shoreline of a glacial lake and transported several miles to where they settled in lake sediments. Today, people excavate these geodes and add them to geodetic and fluorescent mineral collections.
Lamps for viewing fluorescent minerals
The lamps used to find and study fluorescent minerals are different from the newer ultraviolet lamps (called "black lamps"). These new items are not suitable for studying minerals for two reasons: 1) they emit long-wave ultraviolet light (most fluorescent minerals respond to short-wave ultraviolet light); 2) the lamps emit a significant amount of visible light, which interferes with accurate observation, and is not a problem for using the new product.
Scientific lamps are available in different wavelength ranges. Lamps used to study minerals have a filter that allows ultraviolet wavelengths to pass through, but blocks most visible light that would interfere with observation. These filters are expensive and are partly responsible for the high cost of scientific lamps.
For close examination of fluorescent minerals, a 4-watt UV lamp with a small filter window and a small collection of short and long wave fluorescent mineral samples are provided.
UV Lamp Safety
Ultraviolet wavelengths of light are present in sunlight. The length of these waves can cause sunburn. Ultraviolet lamps produce the same wavelengths of light as short-wave ultraviolet rays, which are blocked by the ozone layer of the Earth's atmosphere.
Small UV lamps with only a few watts of power are safe for short periods of use. The user should not look into the lamp or shine it directly on the skin, face of a person, or on a pet. Looking into the lamp may cause serious eye injury. Exposing your skin to UV light can cause sunburn.
Eye protection should be worn when using any UV lamp. Inexpensive UV blocking safety glasses provide reliable protection when using a low voltage UV lamp for short periods of research.
UV lamp protection items used for fluorescent mineral research should not be confused with those sold with "black lamps". These lamps emit low-intensity long-wave UV radiation, which can cause sunburn and eye damage. This is why mineral lamps should be used with more eye protection than black lamps.
Ultraviolet lamps, which are used to illuminate large surfaces of minerals or for outdoor field work, have a much higher voltage than small UV lamps. Eye protection and clothing that covers legs and arms should be worn when working with high-voltage lamps.
Practical use of fluorescence of minerals and rocks
Fluorescence has practical applications in mining, gemology, petrology and mineralogy. The mineral scheelite and tungsten ore typically have a bright blue glow. Geologists looking for scheelite and other fluorescent minerals use ultraviolet lamps to search at night. Scientists in the oil and gas industry sometimes examine drill cuttings and rods with UV lamps. Small amounts of oil in rock wells and mineral grains will glow under ultraviolet light. The fluorescence color may indicate the thermal maturity of the oil. Darker colors indicate heavy oils, while lighter colors indicate light oils.
Fluorescent lamps can be used in mines to identify and track ore-bearing rocks. They have also been used on collection lines to quickly locate valuable pieces of ore and separate them from waste.
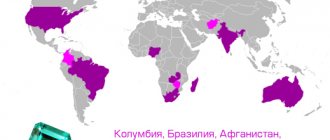
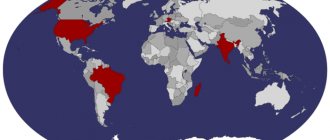
Many gemstones are fluorescent, such as ruby, kunzite, diamond and opal. This property can sometimes be used to detect small stones in sediments or crushed ore and to search for minerals in the area. For example: light yellow diamonds with blue fluorescence are produced by the Premier mine in South Africa, and colorless stones with blue fluorescence are produced by the Jagerfonteen mine in South Africa. The stones mined from these mines are called "Premiers" and "Yagi".
In the early 1900s, many diamond traders were looking for stones with a bright blue fluorescence. They believed that these stones would appear more colorless (less yellow) when viewed under high ultraviolet light. This eventually led to the control of lighting conditions for diamonds when sorting by color. Fluorescence is not usually used in mineral identification. Most minerals do not fluoresce, and this property is unpredictable. A good example is calcite. Some calcites do not glow. And other samples fluoresce in a variety of colors, including red, blue, white, pink, green and orange. Fluorescence is rarely a diagnostic property.
Books on fluorescent minerals
Two excellent introductory books to fluorescent minerals: Collecting Fluorescent Minerals and The World of Fluorescent Minerals are written by Stuart Schneider. These books are easy to understand and each has a fantastic collection of color photographs. The illustrations show fluorescent minerals under normal light and under different wavelengths of ultraviolet light. The books are excellent for learning about fluorescent minerals and serve as valuable references.
Other luminescence properties
Fluorescence is one of several luminescent properties that a mineral can exhibit. Other luminescence properties include:
Phosphorescence
In fluorescence, electrons excited by incoming photons are raised to a higher energy level. Remains there for a split second before returning to its original state and releasing fluorescent light. In phosphorescence, electrons remain in an excited state for a longer period of time before falling. Fluorescent minerals stop glowing when the light source is turned off. Minerals with phosphorescence can glow for a short time after the light source is turned off. Minerals that sometimes phosphoresce include: calcite, celestite, colmanite, fluorite, sphalerite and willemite.
Thermoluminescence
Thermoluminescence is the ability of a mineral to emit a small amount of light when heated. This heating can range from 50 to 200 degrees Celsius - much lower than incandescent temperatures. Apatite, calcite, chlorophane, fluorite, lepidolite, scapolite and some feldspars are sometimes thermoluminescent.
Triboluminescence
Some minerals will emit light when exposed to mechanical energy. These minerals glow when struck, crushed, scratched or broken. The glow is the result of a breakdown of bonds in the structure of minerals. The amount of light released is very small and careful observation in the dark is often required. Minerals that sometimes exhibit triboluminescence include amblygonite, calcite, fluorite, lepidolite, pectolite, quartz, sphalerite, and some feldspars.
Physicochemical characteristics
Fluorite is a unique mineral in that its formula contains fluorine. From the point of view of chemists, it is calcium fluoride.
- The chemical formula is CaF2 .
- Color - blue, purple, red, blue-green, yellow, brownish-yellow, white; happens to be colorless.
- The shine is glassy.
- Transparency - transparent, translucent.
- Hardness - 4.
- Density - 3.18 g / cm3 .
The mineral is fragile and soluble in hydrochloric acid (this releases toxic gas).
It glows under ultraviolet light. If you heat it up, it can glow in the dark.
The heated mineral becomes discolored. When irradiated with X-rays, the color is restored.
Some samples are radioactive, but do not pose a serious danger.
Stones with fluorescence effect
Scientists claim that the number of minerals known to science exceeds 3 thousand units. Approximately 500 of them have the property of fluorescence, including:
- Turitella agates, barites, sapphires, and scapolites glow yellow;
- Blue, green, orange, red can be diamonds, rubies and apatites;
- Blue – fluorites;
- Purple – astrophilites, corundums;
- Violet – calcites, microclines;
- Orange – halites, zircons;
- White – porous limestone;
- Light green – chalcedony.
Photo of orange zircon (left), purple microcline (center), three fluorite stones in the first row and pink calcite (right) glowing in ultraviolet light.
Ultraviolet light is not visible to the naked eye. It is generated by the passage of an electrical pulse inside a sealed flask where mercury vapor is under pressure. If you take a filter to block visible light, then with such a lamp you can go in search of minerals.
Fluorescence and jewelry art
The property of phosphorescence is used in jewelry to identify precious stones. Despite the fact that there are currently no unified standards for measuring fluorescence, its presence is an important diagnostic indicator.
Besides fluorite, a common stone that has the same properties is diamond. In most cases, such optical effects can be observed exclusively in ultraviolet light. Some stones can also glow in the sun.
Diamonds, which are transparent at first glance, can shimmer in multi-colored colors in ultraviolet light: they appear blue, yellow, green, pink. The more intense the effect, the more expensive the stone is.
diamond fluorescence.
Varieties
Fluorite, based on its structure and color, was divided into the following varieties:
- Alpino. Mined in the Swiss Alps. Octahedral crystals have a red color scheme.
- Antozonite (radiofluorite, fluorspar). Radioactive stone of dark purple color. When cracked, it releases a specific odor.
- Derbyshire John (Derbyshire spar, blue John, blue John). It is mined in Derbyshire (UK). Blue colored stones. The structure is usually banded: blue, purple or cyan stripes alternate with greyish, white, yellow.
- Lithos-lazuli . It is distinguished by concentric stripes of red (purple) color.
- Ratovkit . Loose stones of purple color. The origin is sedimentary.
- Chlorophane . Instances that exhibit an intense green fluorescence effect when heated.
Sometimes calcium in fluorite is replaced by other elements. These types of stone are:
- Yttrofluorite (calcium replaced by yttrium).
- Zerfluorite (contains cerium instead of calcium).
Application
Fluorite is a stone used in many fields.
Jewelry
Thanks to its beauty and rich color range, this stone is popular among jewelers.
Jewelry (beads, necklaces, bracelets and sets) made from pieces of different colors look especially impressive.
The frame is selected so that it harmonizes with the color of the stone: cupronickel, silver, “golden” jewelry alloy, irradiated brass.
Arts and crafts
The mineral is malleable in processing. Tableware, souvenirs, and small plastic items are made from it. The bonbonnieres, photo frames and other little things decorated with it are not cheap, so they can be classified as VIP gifts.
They make pyramids, balls, and body amulets from fluorite crystals.
Other areas
The industry uses raw materials of non-jewelry quality:
- Metallurgy. The addition of fluorite lowers the melting point of the ore.
- Chemical industry. Fluorspar is used to make hydrofluoric acid.
- Optics. Colorless transparent specimens are used to make lenses for cameras and night vision devices.
Collectors of mineralogical collections are offered a wide range of this stone:
- agglomerates;
- Druze;
- crystals of various shades and deposits.
Polymer with LEDs
Artificial decor containing LED lighting inside is very effective. They are a hollow, durable structure. There is an electronic circuit inside: a photocell plus an LED. The body of artificial stones can be collapsible or monolithic. In the first case, there is the possibility of replacing parts.
Fluorescent sea stones for reservoirs on site Source avto.goodfon.ru
Paving slabs painted with fluorescent paints Source inventrade.ru
As a result, this type of stone creates a brighter and more colorful backlight. To make paths, materials of different shades of light are often used. Polymer materials have several advantages:
- attractive appearance;
- you can purchase different shapes of stones;
- wide range of sizes;
- looks brighter than previous options.
On a note! In the cold season, LED stones do not hold a charge well, so at night they can create quite dim lighting.
Fluorescent small pebbles for decorating the borders of paths in the yard Source acmelight.biz.ua
Medicinal properties
Fluorite, according to lithotherapists, helps to heal the body.
When using this stone for medicinal purposes, you should not refuse treatment prescribed by your doctor.
The healing properties of fluorite are used if there are the following problems or diseases:
- insomnia, depression, tendency to stress;
- atherosclerosis;
- consequences of stroke;
- kidney diseases;
- weather dependence (aches, migraines);
- eye fatigue, weakened vision (it is especially useful for the eyes to look at the light through a transparent green or blue fluorite crystal);
- aging of the skin (you can rejuvenate it with a massage with fluorite balls or rollers);
- weakening of the immune system (wearing fluorite jewelry will help boost it).
This mineral strengthens joints, bones, teeth and accelerates the regeneration of body tissues after injury or surgery.
How to install glowing stones in the landscape with your own hands
After the luminous material is prepared, it must be installed correctly. For example, if you painted pebbles or crushed stone, then all the material is scattered randomly over the area of the path. When large stones with phosphor are also used to organize street design, they are allocated places along the curbs on the lawn.
Organizing a site in the yard with paving slabs and small luminous pebbles Source stroi-baza.ru
Painted chips are used for painting on the lawn or for filling joints between paving slabs. To ensure that the material is evenly distributed, you need to sweep it and make sure that each side of the tile is filled.
Magic properties
Fluorite, in addition to healing properties, also has magical qualities.
The stone helps the owner to discover and develop abilities and talents, to take a fresh look at himself and the people around him.
Fluorite is important for a person who is trying to develop his analytical abilities. It will help highlight the main thing; its owner will not waste time on secondary issues and problems.
This stone is appreciated by fans of meditation. It is believed that by peering into a fluorite ball, one can see future events.
The mineral (especially green fluorite) is an excellent amulet against negative external influences. Attempts by other people to control the consciousness of its owner will be unsuccessful.
Fluorite takes away not only negative mental energy, but also radiation from gadgets. It is useful to place a stone near a computer, microwave, etc.
The color of fluorite affects its magical properties:
- Blue. Helps unlock creative potential and organize thoughts. Depending on the needs of the body of the owner of the stone, it calms or activates its energy.
- Violet. Ideal for meditation, helps open the “third eye” and also gain sanity.
- Green. Neutralizes negative energy directed at the owner of the mineral and helps heal spiritual wounds.
- Yellow. Strengthens creative and intellectual abilities. Positively affects the microclimate in the team.
- Red. Relieves despondency, depression, encourages creative activity. Such a stone is not suitable for overly aggressive people, and it is not recommended for other people to wear it constantly.
- Colorless. Harmonizes the intellectual and spiritual level, the work of all chakras. Charges a person’s aura with the energy of the Universe.
Who is suitable according to their zodiac sign?
Fluorite has perfect compatibility with two zodiac signs: Virgo and Gemini :
- Virgo, under the influence of the stone, will begin to think about the meaning of life, highlight the main priorities, without wasting time on unimportant activities.
- Gemini will become more attentive and kinder to others, and will begin to understand those areas of life that they considered uninteresting.
According to the horoscope, the magical properties of fluorite are also suitable for the following zodiac signs:
- Aquarius will get stronger physically, demonstrate their abilities and talents in important matters, and achieve success in their chosen field of activity.
- Aries , striving for spiritual self-improvement, will receive help from this mineral.
- Capricorn will feel the urge to be creative.
- Pisces will be able to recharge their energy from the stone so much that they will happily share their vitality and optimism with other people.
The rest of the zodiac signs (except one) can wear jewelry with this mineral, but its magical properties most likely will not affect them.
- for Sagittarius , as it can contribute to the distortion of any information. The magical properties of the following stones are well suited to Sagittarius: lapis lazuli, apatite, chrysocolla, prasiolite, blue sapphire, spinel, ruby, chalcedony, ulexite, chrysoberyl, rhodolite, diamond, amber, sodalite, blue quartz, variscite.
How to wear
Fluorite can be worn in the form of jewelry, key chains, or you can put the raw mineral in your pocket or sew it into your clothes. In order for the stone to remain intact, it must not come into contact with solid objects.
It is believed that the magical properties of fluorite are more pronounced if you wear earrings, rings or rings (preferably on the ring finger). The frame can be gold or silver.
Pendants, necklaces, and beads look beautiful, but they need to be worn infrequently and not for long, otherwise the stone can have a depressing effect on the owner’s psyche and lead him astray. The abundance of stone (for example, in bracelets) will have the same effect on the owner.
Fluorite is suitable for people of any age.
For men, rings, tie clips, and cufflinks are made from it.
The rich color palette of the stone, moderate or strong shine allows women to choose jewelry for any outfit.
Models with fluorescent coating
The material can be made on different bases: granite or marble chips, small crushed paving slabs, crushed stone and others. To create a lighting effect at night, they are simply coated with fluorescent paint. All staining is done by hand. River pebbles and sea stone can also be useful for this purpose.
Glowing stones laid out in a pattern on the path Source landas.ru
Small fluorescent stones for decorating the local area Source fastbox.su
Luminophores are applied with regular paint brushes; a spray bottle can also be useful. In case you need to decorate a dimensional path, it is better to use the pour-on painting method, since manually applying the coating takes a long time.
Remember, in order for the phosphor to adhere well to decorative fragments, you must first wash and degrease them. This design method has its advantages:
- looks good;
- the material is non-toxic;
- lasts a long time even when exposed to moisture.
On a note! Small crushed stone is very difficult to paint by hand, so you can proceed as follows. Pour the dye into a large container, pour out the material and mix thoroughly. The finished raw materials are simply poured into the prepared place. Let it dry.
Price
Fluorite is an inexpensive mineral, so anyone can buy it. Examples of prices in Russian online stores:
- bead (8 mm) - 20 rub. ;
- tumbling (2 cm) - 160 rub. ;
- earrings (brass) - 230 rub. ;
- cabochon (26 x 22 x 11 mm) - 270 rub. ;
- crystal (8 cm) - 708 rub. ;
- intergrowth of crystals - 840 rub. ;
- necklace— RUB 1,163. ;
- rosary (30 grains, d=10 mm) - 1,220 rub. ;
- ball (4.5 cm) - RUB 2,316. ;
- figurine “Owl” (15 cm) — RUB 13,000.