Platinum is a precious metal, the high cost and demand for which is ensured by the minimum coefficient of expansion, electrical and thermal conductivity and high strength of the material.
This metal looks like silver, but its price is higher than gold.
Platinum does not oxidize and is not subject to chemical corrosion , is resistant to acids and alkalis with the exception of “regia vodka” (a mixture of nitric and hydrochloric acids) and sulfuric acid when heated, and exhibits high catalytic properties.
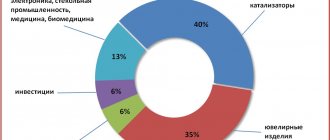
In this regard, platinum is used in the following industries:
- Automotive industry . 40% of platinum is used for the production of afterburning catalysts, especially in European-made cars.
- Jewelry production . 35% of the world's platinum production goes into jewelry, which is traditionally valued for its high durability, quality color and luster. As a rule, platinum in jewelry is present in the form of an 85% alloy with other platinum group metals, improving its malleability and ease of processing.
- Electronics, medicine . 13% of platinum, due to its high electrical conductivity and refractoriness, is used for the manufacture of radio electronics microcircuits. It is also used to make oxygen sensors in cars, which provide the necessary ratios when mixing fuel and air in engines, and are involved in the operation of airbags and climate control. The biocompatibility and chemical inertness of platinum allows it to be used for the manufacture of catheters and electrical stimulators.
- Chemical industry . 6% platinum is used to make catalysts for heavy chemical production processes, for example, for the synthesis of nitric acids. Platinum catalysts are integral participants in the processes of isomerization and reforming of oil, which are used to produce high-octane gasoline components.
Devices containing platinum
Platinum contains:
- automobile catalysts (see photo);
- capacitors;
- microcircuits;
- generators;
- relay;
- other details.
Since the auto industry and electronics are the main industries consuming platinum, when searching for primary secondary raw materials for extracting this metal, it is worth first of all paying attention to the devices produced by these industrial segments.
Let's look at the parts that contain the most .
Automotive catalysts
Among automobile catalysts, the following two types can be distinguished:
- Based on the classical platinum-rhodium-palladium system. The approximate metal content in parts per million per ton of ceramic support is respectively 1470-270-900 for the specified system. The approximate cost of such catalysts is 55 euros per 1 kg .
- Platinum-rhodium systems typical for premium car catalysts. Their platinum content is about 3000 parts per million . The cost of catalysts of this type is estimated at approximately 70 euros per 1 kg .
Knowing these formulas and the type of part, you can calculate how much platinum is in the car catalyst.
Capacitors
20% of the total mass of electronic devices are capacitors.
Depending on the type of capacitor, the amount of precious metals , in particular, platinum in capacitors varies , which determines the final cost of the product when it is purchased.
Below is a table of capacitors with markings and characteristic distinctive features in descending order of their platinum content and, accordingly, cost:
| External sign | Price (%) | Marking | |
| H30 | Thin, green | 100 | 5Н30_68Н_0481, 5_Н30_68Н_1079 |
| 5-D | Thin, green | 78 | 5D/68n/WD |
| H30 | Fat, red-haired | 68 | 4H30_47H_0578, 5H30_33H_0278 |
| 5D | Fat, red-haired | 65 | 4DB_68n_U3, 5D_47n_WD |
| H90 2M2 | Fat, red-haired | 52 | 6B_H90_2m2 |
| H90 1M0 | Fat, red-haired | 50 | 6H90_1M0_0582, 6_H90_1m0_0685 |
| KM, others | Thin, green | 45 | 5М75_1Н2_0572, 4М_Н47К_0375 |
| H90 | Fat, red-haired | 38 | 6_H90_m47_1085, 6BBF_m22_U7 |
Thus, the most valuable are H30 capacitors, which have the highest platinum content.
Other radio components
As for other radio components, platinum is present in:
- Relays , namely, wires and contacts in their composition. Among the most famous relays containing platinum are relays RES-9, RES-10.
- Electronic tubes .
- Microwave devices .
- Generators as part of control and measuring equipment (G2-57, GZ-109).
- Thermoelectric converters TPR .
We talked in detail about the content of various precious metals in radio components here.
Palladium and its uses
Palladium is a chemical element, a precious metal, also silvery white in color. Designated in the table as Pd. It was isolated from platinum ore by the English chemist Wollaston (1803). Palladium is widely used for the manufacture of capacitors, some types of relays, contacts, and in microcircuits. The metal was also used in Soviet times for the production of radio-electronic components, and it is still used today. It is most actively used in the electronic, chemical, military, and aerospace fields. Scientists are conducting experiments to expand the areas of use of this precious metal.
Microcircuits and capacitors contain a palladium-platinum alloy to improve the stability of the functioning of parts in any environment, including at a significant increase in temperature.
Applications of palladium
- Automotive industry. There is an urgent need in this industry to switch to palladium, since it has a lower cost than platinum, which is currently used in catalysts.
- Petrochemical industry. Used as catalysts (in chemical), oil cracking (oil and gas).
- Electrical engineering, radio electronics (for coating capacitors).
- Medicine.
Palladium is a commodity metal, its price on the London Metal Exchange is constantly growing, thanks to the expanding scope of palladium.
We will buy radio-electronic components containing platinum and palladium from you at very good prices.
Methods of extraction from radio components
To extract platinum, the most acceptable from an economic point of view is the collector smelting method , presented in a number of Russian patents RU 245899, RU 2180011.
The technology includes the following stages:
- Grinding of the original scrap.
- Melting scrap in furnaces in the presence of a collector metal.
- Blowing air through the melt to form collector metal oxide and molten platinum concentrate.
The difference in the methods lies in the stages of the processes and the composition of the collector system, which is responsible for the selectivity of the platinum separation process.
Isolation of platinum from electronic waste is also possible using the hydrometallurgical method .
One of the methods is to use the method of hypochlorite leaching of waste and is presented in patent RU 2244759.
The process is carried out at a certain concentration of hydrochloric acid and active chlorine (the source of chlorine is alkali metal hypochlorites) and a temperature of about 65 ° C, with a ratio of liquid to solid phase of 7:1.
When this method was used to recycle ceramic capacitors, the purity of the platinum was approximately 90% .
Another hydrometallurgical method is presented in patent RU 210339. Waste is treated with a mixture of hydrochloric acid, hydrogen peroxide and dimethylformamide (DMF) at a temperature of 100 °C.
As a result of the hydrolytic cleavage of DMF under the conditions used, platinum is reduced, after which DMF is regenerated, and the resulting solution is sent for processing.
In general, both hydrometallurgical and pyrolytic methods can be used to recycle electrical scrap to recover platinum. The same methods are recommended for metal extraction from spent catalysts.
The isolation methods presented above are the result of scientific research , fully or partially implemented in pilot and industrial production after obtaining the appropriate permits to carry out activities in the processing of precious metals.
On our website there is a separate material dedicated to platinum refining.
Radio components containing gold
Gold-bearing deposits are developed if the content of the precious element is at least 1 gram per ton of rock. In one chip there is from 1 to 5 percent yellow metal. The leads of the part, enclosed in a ceramic case, are coated with gold.
If it is made of plastic, the content of valuable raw materials is less - from 0.2 to 1 percent. In transistors, the precious element is about 2 percent. The substrate located under the conductor is made of gold.
But capacitors break all records. Their size is approximately equal to a three-liter jar. One such part contains approximately 8 grams of yellow metal. In addition, there is also 50 grams of silver. However, only capacitors used in military equipment - generators and radio signal transmission stations - are equipped with expensive filling.
Some gold can also be extracted from radio tubes. The precious element is deposited on a grid located near the cathode. The latter, when the lamp is operating, heats the grid. When exposed to heat, it begins to release electrons. This disrupts the operation of the product.
Therefore, the radio component needs to be coated with gold . Spraying from it is also found on the legs of objects of consecration, but this only applies to old samples, decades old.
Several microns of precious raw materials were previously applied to connectors of all types of semiconductors, such as diodes, optocouplers, thyristors, and zener diodes. Gold is rarely found in resistors. However, some of them, along with silver, also contain a little yellow metal.
These are the standards by which radio components were manufactured in the USSR in the 70s and 80s of the last century. Gold is also found in modern radio components . However, it is impractical to extract hundredths of a gram from an item for which tens, or even hundreds of thousands of rubles were paid. Let the old parts be used - that's another matter.
Most often, radio components containing gold are found in old-style computers, switching devices, and radio equipment. Electronic computing units of the SM and CE series should be of primary interest to applicants. One such machine contains from 0.2 to 10 kilograms of gold. Some military equipment can boast of the same thing.
Beginners will find it useful to list not only the general names of radio components equipped with gold “filling,” but also specific model designations. So:
Transistors KT201, KT203, KT3102, KT301, KT306, KT605. All of them are equipped with golden colored legs.
KT802, 808, 803, 809, 812, 908. We need samples produced before 1986. In later models gold was not used.
KT907,904, 606. Externally they have no gold elements and no yellow color. However, valuable raw materials are actually present.
But KT602, 604, 611, 814, 815, 816, 9909 have gold cases.
Relay RES9, 10, 15, 22, 34, RPS24, 32, 34, RKG15.
Microcircuits K142EH, K50, K56PY2, AOT101, K145, also known as the “white spider”.
Chips K133, 134, 178, 249, 564, 565, K140, 157, 217.
Diodes of the D226 series and similar ones.
Capacitors Km3, 4, 5, 6, 10, 11, 12, 13, 14, 15, 16, 17, 52-1, 52-7, K53-1, 53-6, 53-7, 53-10, 53 -15, 53-16.
Resistors PTP1, 2, PLP2, 6, PP3-40, 3-41, 3-43, 3-44, 3-45, 3-47, KSP1, 4, KSU1, KSD1, KPU1, KPP1, SP5-1, 5 -2, 5-3, 5-4, 5-14,5-15, 5-16, 5-17, SP3-19, 3-44.
Connectors SNP59-64V, SNP59-96R, GRPPM7-90Sh, RPPG2-48.
Switches TV1, P23G, Pg2-5, 2-6, 2-7, 2-10, P1T3-1V, PR2-10, PKN8, PT33-26, PP8-6, PPK2.
Sales of waste
To sell electronic waste and autocatalysts in order to obtain financial benefits due to the presence of platinum in them, both individuals and legal entities must contact the appropriate organizations involved in the reception, evaluation and processing of electronic and catalytic scrap for refining industries.
Among such organizations:
- ,
- ,
- NPO "Radiotechnical Precious Metals".
With the help of such companies, it is possible to carry out an expert assessment of the source material using physical and chemical analysis methods for an objective assessment of the cost of scrap and further professional processing of raw materials.
How to distinguish palladium from platinum in radio components
Expert opinion
Vsevolod Kozlovsky
6 years in jewelry making. Knows everything about samples and can identify a fake in 12 seconds
Most platinoids have similar physicochemical characteristics. Among them is the special structure of the atomic lattice, which determines their pleasant, noble shine. Visually, Ag, Pt, Pd are very similar. Therefore, to distinguish them, you will need some reagents. It is most convenient to purchase special probes for qualitative reactions to these metals.
Knowledge of chemistry can help. Palladium, unlike platinum, reacts with concentrated nitric acid, and a drop of HNO3 will acquire a reddish tint.
Why separate metal?
Many chemists use palladium isolated from radio components as a catalyst. Jewelers refine this metal to repair palladium jewelry. Some craftsmen sell the mined precious metal to pawn shops, which falls under Article 19.14 of the Code of Administrative Offenses of the Russian Federation.
Many are attracted by the fact that the cost of Pd on the precious metals market today exceeds the cost of gold. According to experts, this year the price of this element will rise, as demand significantly exceeds supply.
Palladium scrap prices
Scrap prices depend on many parameters - purity, quantity, sample availability, brand popularity. If it is a pure element obtained through refining, there is a chance to sell it for a good price. In radio components, palladium is usually 500 fine, and its cost rarely exceeds 500 rubles per gram.
Reception points can evaluate the product individually, especially if the markings have been preserved. And some parts, such as capacitors, can be purchased by collectors at a cost that is several times higher than the cost of palladium in them. Therefore, before attempting to melt down scrap yourself, consult with specialists.