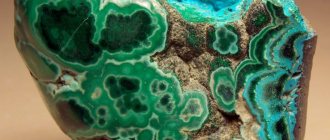
Anhydrite (from ancient Greek ἀν- - a negation prefix and ὕδωρ - “water”, i.e. “devoid of water”) is a mineral from the sulfato class: calcium sulfate. Chemical formula: CaSO4. Glassy, pearlescent luster. Hardness 3-3.5. Specific gravity 3.5 g/cm3. The color is bluish, bluish, white. Crystals are transparent or translucent. The line is white. Crystalline varieties have perfect cleavage in three directions. Solid granular, marble-like or dense masses, less often prismatic, tabular crystals. Rhombic syngony. Hatching is observed on the crystal faces.
Story. Origin
Colorless or slightly colored anhydrite was valued in ancient times. Egyptian and Roman artisans made vases and lamps from it. Sometimes anhydrite tiles were used to line the walls of residential buildings. In the 19th century and early 20th century, writing instruments made from anhydrite were popular.
In 1804, this mineral was studied in detail by the German scientist Abraham Werner, who proposed calling it anhydrite (translated from Greek as “deprived of water”).
After some time, another name for the stone appeared - angelite (from the Greek “aggelos” - angel). This brand of mineral arose because of its color, reminiscent of the blue of the sky, covered with haze. Then, due to the fact that the extraction of blue stones is limited, anhydrites of other colors began to be called angelites: whitish, gray, purple, reddish and milky minerals.
This stone is also called karstenite. This name was given to it because of its origin. The fact is that as a result of dehydration of gypsum, caverns and karsts are formed in its deposits - underground cavities in which anhydrite (karstenite) is found. After all, this mineral is dehydrated gypsum.
Color palette
Most anhydrite samples are gray in color. The mineral can also be colorless or white. There are rare specimens of a bluish tint. Impurities of bituminous organics make karstenite purple or red. Barium and strontium are responsible for giving some specimens pink, brown, and blue shades. Violet-red and red-blue anhydrite looks unusually beautiful.
Photo gallery of colors
Blue(Angelite)
Red
Grey
White
Violet
Physicochemical characteristics
Anhydrite is a modification of anhydrous calcium sulfate. Upon contact with water, the mineral increases in volume and gradually turns into gypsum.
- Chemical formula - CaSO4 .
- Color - white, blue, bluish-gray, sometimes gray-violet, red-violet, red.
- The shine is glassy, greasy, pearlescent.
- Transparency - opaque or translucent; there are also transparent stones.
- Hardness - 3,0–3,5.
- Density - 2.8–3.0 g /cm3 .
It does not decompose in hydrochloric acid.
Anhydrite and gypsum
Both of these minerals, in terms of physical and chemical properties, stand apart from anhydrous and aqueous sulfates, but are closely related to each other in terms of the conditions of occurrence and formation. Therefore, we will describe them together.
The so-called calcium hemihydrate, which is easily produced artificially under natural conditions, has not yet been established with complete certainty.
Anhydrite
- CaSO4. The name of the mineral (“anhydrous”) indicates the absence of water in it, unlike gypsum.
Rice. 236. Crystal lattice of anhydrite
Chemical composition
. CaO 41.2%, SO3 58.8%. Quite often it contains strontium as an impurity.
Rice. 237. Position of sulfur and calcium ions in the anhydrite crystal lattice. The filled circles of calcium ions correspond to those calcium ions that are visible in Fig. 238
singonia
rhombic;
rhombo-bipyramidal c. With. 3L23PC. The anhydrite crystal lattice is shown in Fig. 236 and 237, and the structure model is in Fig. 238. S6+ ions are located at the centers of tetrahedral O2- groups, and each Ca2+ ion is surrounded by eight oxygen ions. It is characteristic that the dimensions b and c in the unit cell are almost exactly the same. However, the structure cannot be considered pseudo-tetragonal along the a-axis, since the location of the ions on the (001) plane is on the left in Fig. 237 - and on the (010) plane - closer to the observer - not the same. The (001) face is centered, while on the (010) face the Ca2+ and [SO4]2- ions do not form horizontal rows. The appearance of the crystals
is thick-tabular or prismatic (Fig. 239).
The faces are often hatched parallel to the a and b axes. Well-formed crystals are rare. Aggregates
. Usually observed in solid granular masses, sometimes in columnar aggregates.
Rice. 238. Model of the crystal structure of anhydrite in the orientation shown in Fig. 237
Color
Anhydrite is white, often with a blue, grayish, sometimes reddish tint.
Colorless transparent crystals are found. The luster
is glassy, on the cleavage planes {001} there is a pearlescent tint. This is especially evident when samples are heated. Ng=1.614, Nm= 1.576 and Np=l.571.
Rice. 239. Anhydrite crystal
Hardness
3-3.5.
Cleavage
along {001} is perfect, along {010} and {100} is average.
Along these three mutually perpendicular directions, cubic chips are quite easily obtained from crystals. Ud.
weight 2.8-3.0 (for transparent differences 2.96).
Other properties
. In the presence of water at atmospheric pressure, it gradually turns into gypsum, greatly increasing in volume (up to 30%). As external pressure increases, this transition becomes more difficult.
Diagnostic signs.
Anhydrite differs from other sulfates of the group under consideration in its smallest specific gravity and directions of cleavage planes, as well as in its optical properties (especially birefringence). It differs from marbled masses of carbonates (calcite, dolomite, magnesite) in that it does not emit CO2 when exposed to acids. It differs from gypsum in hardness (cannot be scratched with a fingernail).
P. p. tr. melts into white enamel, turning the flame a reddish-yellow color. It does not melt with soda and is not absorbed by coal (unlike barite), but it decomposes and produces sulfur liver, which blackens the silver plate. In powder form, soluble in H2SO4. Moreover, unlike anhydrous sulfates of Ba, Sr and Pb, the solution does not become cloudy from moderate addition of water. Slightly soluble in HCl.
Origin
. Huge masses of anhydrite are found in sedimentary rock strata. As a product of chemical precipitation in lagoonal and dying sea basins, anhydrite is almost always accompanied by gypsum, into which it is relatively easily transformed when anhydrite layers reach the surface. It has been established that this transition, according to data from numerous boreholes and mine workings, takes place to a depth of 100-150 m from the surface (anhydrite masses follow below). Obviously, at significant depths, the pressure of the overlying rocks is so great that the increase in the volume of the rock mass, which accompanies the transformation of anhydrite into aqueous sulfate - gypsum, cannot occur.
Very often, anhydrite is found in salt deposits, both in the form of individual crystals and in the form of layers and interlayers, sometimes unusually thin (as thick as a paper sheet), interlayered with halite, sylvite, carnallite, etc.
It is observed relatively rarely in some hydrothermal
and occasionally in contact-metasomatic deposits. It should also be noted that anhydrite has been found in voids among lavas in areas of volcanic activity.
It is very likely that the thick anhydrite strata established in gypsum-bearing areas occurred through dehydration under high pressure of overlying rocks of gypsum strata originally deposited in salt-bearing basins. However, in deeper metamorphic rocks, anhydrite, like other sulfates, is completely absent.
Practical significance
. Anhydrite, like gypsum, is used mainly in the production of binders (cements). Dense fine-crystalline varieties are also used for all kinds of crafts.
Place of Birth
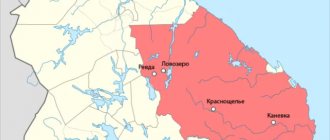
. Large deposits of anhydrite at depth are distributed in gypsum-bearing strata of Permian age along the entire Western Urals, in the Arkhangelsk, Vologda, Kuibyshev, Gorky regions, in the Donbass and other places.
Gypsum
— CaSO4•2H2O. "Gypsum" is the old Greek name for the mineral.
Chemical composition
. CaO 32.5%, SO3 46.6%, H2O 20.9%. Usually clean. In the form of mechanical impurities, the following are found: clay matter, organic substances (odorous gypsum), inclusions of grains of sand, sometimes sulfides, etc.
Rice. 240. Projection of the gypsum crystal lattice onto a plane perpendicular to the c axis. Dashed lines - cleavage directions
singonia
monoclinic; prismatic c. With. L2PC Crystal structure. According to X-ray data, the layered structure of this mineral is clearly visible. Two sheets of anionic groups [SO4]2-, closely associated with Ca2 ions (Fig. 240), form double layers oriented along the (010) plane. In the figure, these layers are located perpendicular to the drawing plane and follow a vertical direction (the boundary lines are shown with dotted lines). H2O molecules occupy spaces between these double layers. This easily explains the very perfect cleavage so characteristic of gypsum. Each calcium ion is surrounded by six oxygen ions belonging to SO4 groups and two water molecules. Each water molecule binds a Ca ion to one oxygen ion in the same double layer and to another oxygen ion in the adjacent layer (see Fig. 240).
Rice. 241. Lamellar crystal of gypsum b{010}, l{111}, m{110)
Crystal Appearance
Due to the predominant development of {010} faces, the crystals have a tabular (Fig. 241), rarely columnar or prismatic appearance.
Of the prisms, the most common are {110} and {111}, sometimes {120}, etc. The faces {110} and {010} often have vertical shading. Fusion twins are common and come in two types: 1) Gallic by (100) and 2) Parisian by (101). It is not always easy to distinguish them from each other. Both of them resemble a dovetail (Fig. 242). Gallic twins (Fig. 243) are characterized by the fact that the edges of the m {110} prism are located parallel to the twin plane, and the edges of the l {111} prism form an incoming angle, while in Parisian twins the edges of the l {111} prism are parallel to the twin seam ( Fig. 244). Aggregates
. In voids it occurs in the form of drusy crystals. Dense, finely crystalline aggregates are common. In cracks, asbestos-like parallel-fiber masses of gypsum with a silky sheen and an arrangement of fibers perpendicular to the walls of the cracks are sometimes observed. In the Urals, such gypsum is called selenite.
Rice. 242. 'Dovetail' - transparent plaster double
In cases where gypsum crystallizes in loose sandy masses, it contains in its environment many trapped grains of sand, clearly visible on the cleavage planes of large crystalline individuals (the so-called Repetek gypsum).
Rice. 243. Gallic twin fused at (100). m {110}, l{111}, b {010}
Color
gypsum white.
Individual crystals are often water-transparent and colorless. It can also be colored grey, honey-yellow, red, brown and black (depending on the color of the impurities captured during crystallization). The luster
is glassy, with a pearlescent sheen on the cleavage planes. Ng = 1.530, Nm = 1.528 and Np = 1.520.
Rice. 244. Paris twin of intergrowth according to (101) l {111}, b {010}, e {103}
Hardness
1.5 (drawn with a fingernail).
Very fragile. The cleavage
along {010} is very perfect.
Ud.
weight 2.3.
Other properties
. It has noticeable solubility in water. A remarkable feature of gypsum is the fact that its solubility, with increasing temperature, reaches a maximum at 37-38°, and then drops quite quickly (Fig. 245). The greatest decrease in solubility occurs at temperatures above 107° due to the formation of “hemihydrate” - CaSO4 • 1/2H2O.
Rice. 245. Dependence of gypsum solubility on temperature
When heated under conditions of atmospheric external pressure, as thermograms show, gypsum begins to lose water at 80-90°, and at temperatures of 120-140° it completely turns into hemihydrate, the so-called model or plaster gypsum (alabaster). This hemihydrate, mixed with water into a semi-liquid dough, soon hardens, expanding and generating heat.
Diagnostic signs
. Crystalline gypsum is characterized by perfect cleavage in one direction and low hardness (scratchable with a fingernail). Dense marble-like aggregates and fibrous masses are also recognized by their low hardness and the absence of CO2 bubbles when wetted with HCl.
P. p. tr. loses water, splits and fuses into white enamel. On coal in a reducing flame it gives CaS. It dissolves much better in water acidified with H2SO4 than in pure water. However, at H2SO4 concentrations above 75 g/l, the solubility drops sharply. Very slightly soluble in HCl.
Origin
. Gypsum is formed in natural conditions in various ways.
It is deposited in significant masses by sedimentary
through lake and sea salt-bearing dying pools. In this case, gypsum, along with NaCl, can be released only in the initial stages of evaporation, when the concentration of other dissolved salts is not yet high. When a certain concentration of salts, in particular NaCl and especially MgCl2, is reached, anhydrite and then other, more soluble salts will crystallize instead of gypsum. Consequently, the gypsum in these basins must belong to earlier chemical sediments. Indeed, in many salt deposits, layers of gypsum (as well as anhydrite), interbedded with layers of rock salt, are located in the lower parts of the deposits and in some cases are underlain only by chemically precipitated limestones.
Very significant masses of gypsum result from the hydration of anhydrite
in sedimentary deposits under the influence of surface waters under conditions of low external pressure (on average to a depth of 100-150 m) according to the reaction: CaSO4 + 2H2O=CaSO4•2H2O. In this case, a strong increase in volume occurs (up to 30%) and, in connection with this, numerous and complex local disturbances occur in the conditions of occurrence of gypsum-bearing strata. In this way, most of the large deposits of gypsum on the globe arose. In the voids among solid gypsum masses, nests of coarse, often transparent crystals (“spary gypsum”) are sometimes found.
In semi-desert and desert areas, gypsum is very often found in the form of veins and nodules in the weathering crust
rocks of the most varied composition. It is also often formed on limestones under the influence of water enriched with sulfuric acid or dissolved sulfates. Finally, it is found in oxidation zones of sulfide deposits, but not in such large quantities as might be expected. The fact is that in the overwhelming majority of cases, sulfide ores contain pyrite or pyrrhotite in varying quantities, the oxidation of which (especially the first) significantly increases the content of sulfuric acid in surface waters. Water acidified with sulfuric acid significantly increases the solubility of gypsum. Therefore, in a number of deposits, gypsum is more common in the upper parts of primary ore zones, where it occurs in cracks along with other sulfates.
Relatively rarely, gypsum is found as a typical hydrothermal
mineral in sulfide deposits formed under conditions of low pressure and temperature. In these deposits it is sometimes observed in the form of large crystals in voids and contains inclusions of chalcopyrite, pyrite, sphalerite and other minerals.
Pseudomorphoses of calcite, aragonite, malachite, quartz and other minerals based on gypsum have been repeatedly established, as well as pseudomorphoses of gypsum based on other minerals.
Practical significance
gypsum is great, especially in construction.
- Model or molded (half-burnt) gypsum is used to produce castings, plaster casts, molded decorations of cornices, plastering ceilings and walls, in surgery, paper production for the production of dense white grades of paper, etc. In the construction industry, it is used as cement for brick and stone masonry , for printed floors, making bricks, slabs for window sills, stairs, etc.
- Raw (natural) gypsum is used mainly in the cement industry as an additive to Portland cement, as a material for sculpting statues, various crafts (especially Ural selenite), in the production of paints, enamels, glazes, in the metallurgical processing of oxidized nickel ores, etc. .
Place of Birth
. Sedimentary deposits of gypsum are distributed throughout the globe and are confined to sediments of various ages. We will not stop at listing them. We will only point out that on the territory of the USSR, powerful gypsum-bearing strata of Permian age are distributed throughout the Western Urals, in the Bashkir and Tatar Autonomous Soviet Socialist Republics, Arkhangelsk, Vologda, Gorky and other regions. Numerous deposits of Upper Jurassic age are established in the North Caucasus, Dagestan, Turkmenistan, Tajikistan, Uzbekistan, etc.
Place of Birth
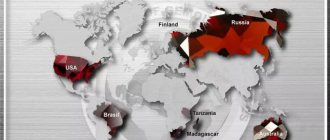
There are not very many anhydrite deposits on the planet; they are concentrated in Europe and America.
Significant reserves of this stone are located in Peru, the USA, Switzerland, and Germany. There are deposits in Chile, Mexico, Canada, Iran, Italy, Russia (for example, the Norilsk Anhydrite mine; the subpolar Urals). Peruvian anhydrites began to be found since 1987 , they accompanied other minerals: calcite, halite, dolomite, gypsum, etc. Minerals from Peru are very valuable: they have a soft blue color, high density, the size of these fine-grained aggregates is more than 10 cm .
The influence of the mineral on the owner and the atmosphere of the house
Anhydrite figurines used to decorate homes are becoming increasingly popular. Products help to attract good luck to the inhabitants of the house, enhance the positive character traits of household members, establish peace and harmony, a warm and friendly atmosphere.
The stone is under the protection of the Moon, so it needs regular recharging to restore its magical properties. On a full moon, it is recommended to place a figurine or pebble on a piece of fabric, preferably purple, and place it on the windowsill. By morning the mineral will return to its original properties.
Application
Anhydrite is used in several applications. Each of them requires raw materials of a certain quality.
Jewelry
According to the jewelry classification, angelite is an ornamental stone, but single craftsmen make jewelry from this mineral. True, due to the fragility of the stone, their range is limited: beads, pendants, pendants, earrings, brooch inserts. Products that experience mechanical stress (cufflinks, rings, bracelets) are rarely made from angelite.
In jewelry, angelite is sometimes combined with gems comparable in jewelry value, for example, jasper, quartz, rauchtopaz, serpentine, and coral.
Decor
This is the main area of use of angelite. Master stone-cutters value the mineral for its beauty, decorativeness, and ease of processing. They make small plastic items, eggs, pyramids, boxes, and tableware from it. Protective amulets made of angelite and figurines of angels are especially popular.
Collecting
Collectors of mineralogical collections appreciate unusual stone specimens. Intergrowths of reddish or blue anhydrite with other minerals: gypsum, dolomite, calcite, halite are especially in demand.
Other areas
Anhydrite arrays of ordinary characteristics are the source material for interior cladding.
Crumbs and other substandard materials are used in the production of cement, sulfuric acid and other sulfur compounds.
Farmers use anhydrite (along with gypsum) to cultivate saline lands.
Jewelry with mineral
Anhydrite, as a jewelry raw material, is little known within Russia. Abroad, on the contrary, this mineral is successfully used to make jewelry.
The most popular is blue Peruvian anhydrite, also known as angelite. Italians love this mineral - the stone can be successfully polished, making beautiful beads and cabochons. Products with this stone look gentle and sophisticated. Prices for such jewelry are more than affordable:
- a pendant made of Peruvian stone starts from 450 rubles;
- earrings can be bought for 800-900 rubles.
Beads from Angelite
Beads for needlework are sold even cheaper. Handmakers use them to create beads and bracelets.
Do you know that the name “angelite” came only in 1987? This is purely a trade name for one of the varieties of anhydrite. In the magical world, angelite is considered a heavenly stone. When a person needs help, it is necessary to turn to the stone with the following words: “My angel, be with me. You are ahead, and I am behind you."
Due to the rarity of the mineral, as well as its fragility, jewelry with angelite is rare. The frames are inexpensive metals - bronze, cupronickel, brass, and occasionally silver. Carved figurines or icons in the form of angels are popular among Catholics.
Also read: Morion - a mysterious black crystal
Medicinal properties
Anhydrite shows its healing power in the prevention of diseases. Due to the presence of sulfate ions in the stone, the following healing properties appear when worn:
- strengthening the immune system, tidying up the human aura;
- resistance to infections;
- accelerating recovery of health after serious illnesses.
Only natural anhydrite has medicinal and magical significance for humans, and the stone must be whole.
It is believed that the mineral can relieve its owner from toothache and alleviate migraines. To do this, it is better to wear it as an insert in earrings.
Lithotherapists claim that anhydrite helps forgetful people increase attention and improve memory.
They also advise drinking 1 glass of water daily, in which this stone was kept for several hours. If you do this regularly, the body will be cleansed of toxins.
Mineral in lithotherapy
Healers and lithotherapists are confident that crystals spread special energy fields around themselves and, when in contact with the body, transmit vibrations to all cells of the body, forcing them to activate hidden resources, which leads to recovery.
Anhydrite helps cope with a number of diseases:
- Fever;
- Toothache;
- Migraine;
- Diseases of the intestinal tract;
- Bronchitis and throat diseases;
- Problems with the thyroid gland;
- Suppuration in the ears, eyes, lungs;
- Alzheimer's disease.
Place the mineral directly near the problem area. Earrings made of anhydrite will help against migraines and toothaches; to treat the thyroid gland and throat, you need to purchase an anhydrite pendant. A ring with anhydrite is needed for diseases of the gastrointestinal tract. Those suffering from Alzheimer's disease, simply absent-minded people who need to improve their memory, should carry this stone with them. Surely Chinese students turn to anhydrite for help during exam periods. Healers advise drinking water charged with anhydrite. To do this, throw a stone into a container of water. By drinking a glass a day, you will gradually rid your body of toxins.
If you are a yoga lover, place an anhydrite crystal next to you when meditating, as it dissolves “energy congestion and traffic jams” and promotes muscle relaxation. In medical terms, the anhydrite mineral improves blood flow even at the capillary level - the muladhara, svadhisthana, and anahata chakras will open.
To restore hormonal balance, lithotherapists recommend massage with polished anhydrite. To do this, you need a few pieces of mineral and a competent massage therapist.
Magic properties
Opinions differ about the magical properties of anhydrite.
Angel Stone
Magicians consider these gems to be “bridges” between people and guardian angels. To establish contact with his heavenly patron, the owner of the stone needs to read a spell or ask an angel to fulfill a wish.
The Guardian Angel fulfills only one wish transmitted through this gem. For the next one you need another pebble.
Christian priests believe that such an influence of the mineral does not exist, since blue anhydrite is called angelite by marketers in order to be more attractive to buyers.
Western esotericists advise recharging this stone under the rays of the Sun, the light of the Moon and stars, so that the angelite quickly gets used to the owner and the world of light.
Other options
The magic of anhydrite also manifests itself in a more mundane sphere:
- Helps create balance between the physical body and aura.
- Calms excessive emotions, removes nervousness. A person does not pay attention to minor troubles and begins to simply enjoy life.
- Helps shy people express themselves in communication, business, and creativity.
- With its help, an overly self-critical owner will be able to forgive himself for past mistakes and get rid of self-criticism.
- Aggressive and callous people will become more loyal to others.
- The stone tells you how to solve problems, get out of difficult situations, and also how to start a new project.
Esotericists believe that this gem establishes a connection with the wisdom of the Universe, with true knowledge.
Some claim that the magical properties of angelite can help one gain the ability to communicate over a distance. Angelite is considered the embodiment of goodness, so the owner of the mineral must be a person with positive qualities. If he is not ready to work on himself in order for negative character traits to disappear, then it is better not to wear this stone, otherwise punishment from higher powers is possible - this is what some esotericists say.
A talisman or amulet in the form of a bird (swan, nightingale, stork) will attract love and friendship to the owner of the gem, and relieve fatigue and irritation.
Restrictions
Lithotherapists recommend not wearing jewelry with angelite all the time. Although the influence of this stone is positive, it manifests itself too persistently, so the owner may become disorganized and childish.
It is best to take this mineral with you or wear jewelry with it before important events to remain calm and balanced.
Scope of use
Anhydrite is one of the components in the production of ammonium sulfate and in the production of sulfuric acid. As a fertilizer, the mineral has found application in agriculture. Anhydrite is also used as an ornamental stone. Due to its different colors, figurines are made from it; inkwells were previously made from it.
In terms of hardness, the mineral occupies an intermediate place between such ornamental hard minerals: jasper, jade, agate, and soft ones: selenite and calcite. Until the 17th century, anhydrite was brought to Russia from different parts of the world. In 1725, by decree of Peter I, the first lapidary production was opened in Peterhof for the production of various crafts from anhydrite: decorative elements, sculptures, vases, etc.
Due to its astringent qualities, anhydrite has found application in construction work as plaster and screeds. To do this, building lime is added to the anhydrite. After 25 minutes the solution sets and becomes solid in no more than 12 hours. It is used in the construction of internal structures.
In Russia, anhydrite is unknown to few people. In Europe, stone is more popular; for example, blue stone is easily polished and is successfully used in Italy, replacing marble.
Anhydrite is mined in large volumes and has a low price. The mineral has a pleasant color, from white to bluish. It is easy to polish.
Healing properties
The active energy of the stone can dull headaches and toothaches, as well as help in the treatment of fever. It is recommended to wear the mineral in the ring to prevent gastrointestinal diseases. Earrings with anhydrite will relieve pain during migraine attacks. For diseases of the throat, thyroid gland and bronchi, it is recommended to wear a pendant.
The magical powers of the stone
The energy of anhydrite is the same as that of jasper, selenite and jade. In China it is compared to the magical power of these minerals. As selenite, it allows you to neutralize the negative effect of the Moon and, conversely, attract a complete positive effect: it extinguishes excitement, a feeling of fatigue, and pacifies the angry state of the owner of the stone and those around him.
Anhydrite has the same properties that jade has: it forces its owner to be decent, conscientious, and courageous. Anhydrite has qualities inherent in jasper - this is the ability to protect against the evil eye. The owner of the stone gains the ability to establish relationships with the right people.
Having universal abilities, anhydrite is not recommended to be worn constantly. Experts believe that continuous guardianship can have a negative impact on the owner of the stone, making him careless, lazy, and infantile.
The influence of the mineral on the owner and the atmosphere of the house
Over the years, people have learned to take advantage of the active properties of stone, using them to their advantage. The house was decorated with figurines or simply mineral crystals. If an important meeting was coming up, then, for good luck, they took the product with them.
Anhydrite does not belong to any specific zodiac sign. You need to know that the mineral is associated with the Moon. To charge it, certain steps must be performed: The stone must be placed on a piece of purple silk, under the moonlight. This must be done during the full moon, so it will recharge with energy.
An anhydrite amulet for each purpose looks like a specific figurine:
- To attract a loved one - a stork or a swan;
- Get a good attitude from management - rabbit and squirrel;
- Images of large animals are suitable for career growth: wild boar, bull, bear;
- Attracting money - toad, fish, sailboat, turtle.
A polished stone kept in the home attracts good luck and success, and can also inspire creative inspiration in the owner and develop positive character traits. Few people know what anhydrite looks like; it is often confused with jasper, chalcedony, and quartz. This allows the mineral to be used as a replacement for these stones, while remaining itself.
Who is suitable according to their zodiac sign?
Anhydrite has light energy, it has a beneficial effect on all signs of the zodiac. The gem is especially suitable for Virgo and Pisces . With its help, the personality potential of representatives of these signs will be fully revealed.
Under the influence of angelite:
- Cancer will be able to become more confident in their strengths and abilities.
- Overly emotional Geminis will become calmer.
- Leo will moderate his ardor.
- Scorpio will be more balanced.
- Sagittarius will feel a surge of energy.
- Aries and Libra will find faithful partners in business.
- Aquarius will not commit rash actions and this will protect them from failures.
- For Taurus and Capricorn , angelite will become an effective talisman of good luck.
Astrological affiliation
Astrologers have not found any contraindications for wearing anhydrite by any zodiac family. It is believed that this mineral is not picky regarding constellations, but does not tolerate certain personal qualities - deceit, hypocrisy, anger.
(“+++” – the stone fits perfectly, “+” – can be worn, “-” – is strictly contraindicated):
| Zodiac sign | Compatibility |
| Aries | + |
| Taurus | + |
| Twins | + |
| Cancer | + |
| a lion | + |
| Virgo | + |
| Scales | + |
| Scorpion | + |
| Sagittarius | + |
| Capricorn | + |
| Aquarius | +++ |
| Fish | +++ |
The mineral will reveal itself best among owners born under the signs of Pisces and Aquarius.
In order for the mineral to work at full strength, anhydrite must be nourished with lunar energy, with which it is closely connected. The gem is charged in the light of the full moon on a piece of purple silk.
Is this stone right for you?
How to wear and care
When caring for this stone, you should take into account its properties (hydrophobia, fragility, low hardness):
- The main rule is to protect anhydrite from moisture. You cannot wear jewelry with it to the beach, to the pool, or in rainy weather. You can’t even wear it on your naked body, as sweat will gradually destroy the pebble.
Anhydrite that has absorbed moisture turns into gypsum.
- The stone must be protected from mechanical stress.
- It should be stored in a separate tightly closed box or box.
- Cleaning with abrasives and strong chemicals is prohibited.
- This gem cannot be cleaned with water. To prevent dirt from accumulating, after use you need to wipe it with a slightly damp cloth and immediately wipe it dry.
Moisture that penetrates into the stone changes its structure, increases its volume and, as a result, turns it into cracked gypsum.
How to distinguish from a fake
Anhydrite is often replaced with plastic. To avoid buying a fake, you need to:
- Carefully examine the gem. Natural stone is rough and has inclusions of other rocks. Glassy veins are often present. The surface is almost always chipped or cracked.
- Remember that a fake can be identified by uniform color or pattern and a very smooth surface.
- Hold a pebble in your hand: natural stone will take a long time to heat up, while plastic will heat up almost immediately.
- Estimate weight: the stone is heavier than the plastic fake.
The authenticity of anhydrite is of particular importance for a person who wants to make the stone his amulet or talisman.
In salons or shops of esoteric paraphernalia you can buy anhydrite, equipped with a certificate of quality.
Runet sites offer jewelry made from this mineral, made by hand-made craftsmen, as well as collectible material.
Price
The cost of angelite or products with it in Russian online stores is relatively affordable (price in rubles) :
- stone from Peru (67x48x39 mm, 200 g) - 2500;
- ball (65 mm) — 6100;
- bead (4–14 mm) — 25–105;
- silver earrings) - 3200;
- pendant (50 mm, silver, leather cord) — 1500.
Jewelry is offered by craftsmen in a single copy, or their quantity is limited.
Read also: Belomorite - a stone donated by the North