Chemical composition
Barium oxide (BaO) 65.70%, sulfur oxide (SO3) 34.30%.
Strontium, lead and calcium can replace barium to some extent, and there is probably complete isomorphic miscibility between Ba and Sr; the intermediate members are called barytocelestine. Angleso barite, or hocutolite , is a lead barite containing approximately 18% PbO. Foreign impurities are sometimes identified as Fe2O3, clayey, organic and other substances.
Varieties
Barite cockscomb = a type of barite
Barytopisolith, redundant name = a type of barite
Barite Ba[S04|. The name is of Greek origin and means “heavy.”
Crystals
Crystallographic characteristics
Barite syngony . Rhombic.
Symmetry class . Rhombic bipyramidal - mmm c. With. 3L23PC. Etc. gr. Pnma(D162h). a0 = 8.85, b0 = 5.43; с0 = 7.13. Axle ratio. 1.627: 1: 1.310, Z = 4.
Crystal structure
Anionic complex of GBOL2- with Ba.
The Ba2+ and S6+ ions are located at distances of 1/4 and 3/4 of the height b (see numbers). The SO4 groups are not quite regular tetrahedra and, as can be seen from the numbers, are oriented differently. Each Ba ion is surrounded by twelve oxygen ions belonging to seven different SO4 groups.
Main forms : Main forms: c {001}, t {210}, z {211}, p(110), d {101} and o {011}, (001) D (101) = 38°51′, ( 001) D (011) = 52°42′, (210) D (210) = 78°20′, (001) D (211) = 64°18′.
Story
The first specimens of the mineral were discovered in the vicinity of the Italian city of Bologna. And until 1800 it was called “Bolognese stone”.
But the stone went down in history under the name proposed by the German mineralogist Dietrich Karsten. The basis is massiveness: the Greek “βαρύς” means “heavy”. Thanks to this property, the stone can be easily distinguished from synthetics and many minerals.
The first area of application of barite was military affairs. These were the “bullets” that were used to load crossbows almost a thousand years ago.
Form of being in nature
The appearance of crystals . Usually found in the form of well-shaped, thin- and thick-table crystals. due to the development of the {001} face. Less common are prismatic, columnar, usually formed by dominant faces of {011} or {101} prisms in combination with {001}, and isometric crystals. Often extremely rich in combinations. Characteristic are split crystals, sometimes having a “rose” shape.
Are doubles rare? Polysynthetic twins are usually observed, causing streakiness on the faces.
The aggregates are often granular, less often platy, dense, cryptocrystalline, and earthy. They are also observed in the form of stalactites and other “sinter” forms with a concentrically zonal structure. Spherical and ellipsoidal nodules with a radial structure are known. In the voids it is often possible to observe spectacular druses of small crystals and brushes in the fracture of which the cleavage of their constituent barite grains is clearly manifested.
Physical properties of barite
Optical
Color. Colorless, water-transparent crystals are found. For the most part, barite, due to foreign impurities, is colored white or gray (microscopic inclusions of gases and liquids), red (iron oxide), yellow or brown (probably iron hydroxides), dark gray and black (bituminous substances), sometimes bluish, greenish and other shades; often zonal.
The line is white.
Shine. Glass.
The sheen on the {010} cleavage planes is pearlescent.
Sheaf-shaped (split) barite crystals and cubic fluorite crystals. Caucasus, Belorechenskoye field
Transparency. In thin chips it is translucent and transparent.
The refractive indices are Ng = 1.648, Nm = 1.637 and Np = 1.636.
Mechanical
Hardness 2.5 - 3.5. Fragile.
The density is high - 4.3-4.7.
Cleavage is perfect along {001}, good along {210}, both directions correspond to weak bonds in the structure, and imperfect along {010}. It differs from the cleavage of calcite by the right angle between the planes; the mineral forms cleavages along a rhombic prism.
The fracture is uneven, stepped.
Chemical properties
Behavior in acids. In powder form, it dissolves slowly in concentrated sulfuric acid; When water is added, the solution becomes cloudy due to the precipitation of BaSO4.
Other properties. Some varieties fluoresce, thermoluminesce and phosphoresce.
Baryte with pyrite powder. Caucasus, Belorechenskoye field
Diagnostic signs
Similar minerals. Celestine, aragonite, calcite, feldspars, anhydrite.
It is recognized by the highest specific gravity among light minerals (barite has the highest specific gravity, only anglesite has a higher density), and the tabular appearance of the crystals. Characterized by insolubility in HCl even when heated (unlike all carbonates). It differs from some silicates similar to it in cleavage (perfect cleavage in one direction) and other characteristics in significantly lower hardness. Without chemical reactions it is difficult to distinguish from celestine.
Associated minerals. Calcite, fluorite, quartz, siderite, hematite, pyrite, galena, etc.
How to distinguish from a fake?
Real barite is very fragile and difficult to process, but there is a demand for it. It is for this reason that you can find a large number of fake stones on the mineral market.
You can distinguish natural barite from a fake crystal by several main features:
- Weight. Barite has a high density, so if you put it on your palm, you can feel a pleasant heaviness. The glass used to imitate this stone weighs much less.
- Contact with fire. If you bring a lit match to natural barite, its flame will acquire a yellow-green hue.
- Susceptibility to mechanical damage. Real barite is easily scratched by a metal object.
To protect yourself from purchasing fake barite, you should make purchases in trusted stores and at the same time require a quality certificate for a product with this gem.
Origin and location
Barite is the most important mineral of barium, with which strontium is closely associated in geochemical processes. According to Goldschmidt, the barium content in the earth's crust is 430 g/m, i.e., more than twice the strontium content, which reaches 150 g/t. Both elements are typically lithophilic, like other alkaline earth elements. The barium cation Ba2+ is almost the same in size (1.35 A) with the potassium cation K+, as a result of which it becomes possible for it to enter into the structures of potassium silicates, such as feldspars, micas, etc. The strontium cation is smaller in size (1.27 A) and more easily replaces calcium, as a result of which plagioclases, as a rule, are richer in strontium, while potassium feldspars are richer in barium. Celsian is the barium analogue of feldspars. Barium and rubidium act as antipodes in potassium feldspars and are considered the most convenient elements for determining the degree of differentiation of mineral formation processes. In alkaline rocks, barium often combines with titanium to form minerals such as benitoite, leucosphenite, cappelenite, etc. Pegmatites contain a small amount of barium, while hydrothermal deposits are noticeably enriched in barium, which is concentrated in barite and, less commonly, barium carbonate (witherite) or in barium zeolites.
Under exogenous conditions, barium and strontium migrate in the form of soluble bicarbonates, while a significant amount of barium still remains on land due to the low solubility of its salts, while most of the strontium follows the migration of calcium and accumulates in seawater. Small amounts of barium also enter marine basins and are deposited there as barite or witherite nodules among sedimentary formations. Sedimentary rocks in general contain more barium than igneous rocks, hence the obvious possibility of mobilizing and enriching hypogene solutions with barium, which were initially poor in this element.
Barite is formed in nature in various ways, but only under conditions of increased partial pressure of oxygen and at relatively low temperatures. Therefore, like all other anhydrous sulfates, it does not occur as an igneous mineral in igneous or deep-seated metamorphic rocks.
It is quite common in hydrothermal As a satellite, it is installed in many deposits of sulfide, manganese (with manganite, braunite), iron (with siderite, hematite) and other ores. Gold-barite veins are known. There are almost pure barite, barite-calcite, barite-fluorite veins with a small admixture of quartz and rare sulfides (galena, sphalerite, chalcopyrite, sometimes cinnabar, etc.). Barite, in small quantities, mainly in the form of nodules, is also common in sedimentary rocks , but under different conditions than anhydrite, gypsum and celestite. For example, it is never found in salt deposits, it is extremely rare in limestones, but it is often found in sedimentary deposits of manganese (in oxide and carbonate ores), iron, in clayey, sandy and other sediments of coastal zones of the seas. This is explained by the fact that soluble barium salts brought from land by surface waters form practically insoluble barium sulfate at the first meeting with [SO4]2– ions in sea waters. Barite nodules are found among muds and in modern seas.
In weathering zones of rocks and ore deposits in areas with a dry climate, upon careful study, small crystals of barite, often columnar in appearance, are often found in association with gypsum and iron hydroxides.
Barite is a chemically stable mineral; therefore, it is found in eluvium, often in large fragments, as well as in concentrates obtained by washing placers. However, like all minerals that have good cleavage and low hardness, as they move into the placer they quickly become crushed and gradually disappear.
Barite deposits
Of the numerous barite deposits in Russia, we will mention only the hydrothermal deposits Belorechenskoye (Krasnodar Territory) and Dzhalankol (Karachaevo-Cherkessia).
In Western Georgia there are a number of vein barite deposits in Kutaisi, Bolnisi and other regions, among tuffs and effusive rocks (porphyrites). Solid barite masses with a symmetrically banded or colloform structure contain impurities in the form of calcite, a very small amount of quartz, and occasionally pyrite, chalcopyrite and galena.
On the Mangyshlak Peninsula (Western Kazakhstan), in the Aurtash deposit, which is large in terms of reserves, barite of sedimentary origin forms a layer in limestone up to 0.5 m thick, distributed over a huge area at a depth of up to 3 m.
The deposits of the Karakalinsky region in Turkmenistan (in the Kopetdag mountains) are also represented by a whole series of veins in sedimentary rocks (sandstones, shales, etc.). In a number of veins (Arpaklen) barite is in association with witherite - Ba[CO3], which in some places develops pseudomorphically along the barite.
Among the largest foreign barite deposits is Meggen on the river. Lenne in Westphalia (Germany). Here, a plateau-like deposit of enormous length (up to 7 km), in places accompanied by a deposit of sulfides, mainly pyrite, is located on the border between the Middle and Upper Devonian sediments. The origin of this deposit is not exactly clear. In addition, Freiberg, Saxony, and Clausthal are known in Germany.
Together with limonite and pyrolusite in the Ore Mountains, Stolberg, Kyffhäuser (Harz), Schmalkalden, Ilmenau (Thuringian Forest).
England (Westmoreland, Cumberland.
USA - Cheshire in Connecticut, Missouri, Wisconsin, central Oklahoma. Beautiful barite crystals and roses are known at Stoneham in Colorado.
Canada (Hants County in Nova Scotia, Madoc in Ontario).
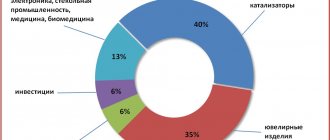
Practical use
Barite is widely used in various industries. It is used in pure form and in the form of barium preparations.
- In the form of a finely ground powder, it is added as a weighting agent to the composition of “clay solutions” used for cementation of loose rocks when drilling oil deposits in order to combat gas emissions and strengthen the walls of wells.
- In the chemical industry it is a raw material for the production of various salts and preparations used in pyrotechnics, tanning (to remove wool), sugar production, in the manufacture of photographic paper, in ceramics for the production of enamels, for the smelting of special glasses with a high refractive index, in medicine and etc.
- In the rubber and paper industries it is used as a filler and weighting agent (wallpaper, oilcloth, linoleum.
- In the paint and varnish industry it is used for the production of high-grade white (mixed with ZnO and ZnS) (lithopone), colored paints, etc.
- As the main component of plaster for the walls of X-ray laboratories, it is used to protect workers from the harmful effects of X-rays.
- Barium metal is used to make some radio tubes.
Barium salts are used in medicine and agriculture (insecticides).
How to care
Barite jewelry
Like any fragile mineral, barite requires delicate care and storage. Basic rules that must be followed to preserve the original appearance of the stone:
- Store it in a separate box with soft walls inside.
- Protect the gem from mechanical damage - impacts, chips, scratches, excessive squeezing.
- Do not leave the mineral in direct sunlight or in places of high humidity.
- To wash, use a mild soap solution, but do not rub the stone with brushes or rough cloths. To dry items with stone, use a soft flannel cloth.
It is also not recommended to allow barite to come into contact with cosmetics, perfumes, as well as cleaning and washing chemical solutions.
Physical research methods
Differential thermal analysis
The main lines on the radiograph: 3.456(6) - 3.058(7) - 2.106(10) - 1.526(6) - 1.259(6) - 1.093(6).
Ancient methods. Under the blowpipe it cracks and fuses only in thin fragments along the edges, and the flame turns yellow-green (characteristic of barium). It is fused with soda on a platinum plate into a transparent mass, which upon cooling becomes cloudy (when fused on charcoal, this mass spreads and is absorbed inside). Unlike celestine, barium sulphide, obtained in a reducing flame of a p.p.t., after wetting with HCl, colors the flame not carmine red, but yellow-green.
Zodiac signs
As an astrological talisman, barite is recommended for the signs of the fire element (Leo, Aries and Sagittarius) to curb and overcome such characteristic qualities as cowardice, laziness, hot temper and unjustified aggression. Constant contact with the stone helps them find peace and confidence in the future.
The effect of the mineral on representatives of the elements of Water (Scorpio, Cancer and Pisces) and Air (Aquarius, Libra and Gemini) is not so noticeable, since all these signs are by nature practical, prudent and sensible individuals.
Barite is neutral for the Earth signs Virgo and Taurus, giving special attention to Capricorns. The stone favors them and welcomes all endeavors. Back to contents