Gold refining is a process of purifying the precious metal from impurities, carried out both in an industrial environment and in a home laboratory. In 2014 alone, 12.5 tons of gold were extracted from electronic waste in Russia. As a rule, gold is refined on an industrial scale by electrolytic means, which involves depositing pure metal on a cathode and separating impurities in the form of sludge. This method is good for its low cost and high degree of refining of the resulting product. Such gold will have a purity of at least 999.9%.
However, there are alternative cleaning methods that some craftsmen use at home. These include dry cleaning of scrap with chlorine and wet cleaning with various chemicals. When molten metal is exposed to chlorine, all impurities sublimate, forming chlorides. While silver chloride, on the contrary, rises and covers the gold. As a result, gold is obtained with a purity of 996.5%, and silver - 999.0%. Wet methods are used to purify gold by first dissolving it and accompanying metals in aqua regia, and then successively treating them with various reagents to precipitate them.
Existing gold purification methods
The following methods are used for cleaning:
- Dry metal refining methods involve treating scrap gold with chlorine or potassium nitrate (KNO3). Nitre purification is a method in which the molten raw material is exposed to the open flame of a burner in a heat-resistant ceramic crucible. Saltpeter is constantly added to the melt in small doses until it no longer causes a reaction from impurities. Borax is used to remove impurities of zinc, bismuth and others. Chlorine purification using the Miller method is a method in which impurities are sublimated to form chlorides. It is usually used in industrial enterprises because it is hazardous to health. It is not recommended to use it at home.
- Wet gold purification methods are designed for processing scrap with various inclusions of other metals. They differ depending on what metals are included in its composition. For example, if there is a lot of silver in the alloy, which prevents it from dissolving in aqua regia, then copper can be added. Wet methods include gold leaching using NaCN.
- Chemical methods for extracting metal from laboratory and technical residues. There are known methods for precipitating gold from a chloride solution using sugar, ethyl alcohol, oxalic or formic acid, sulfur dioxide, sodium thiosulfate, etc.
- The reduction method, based on the inertness of gold to the effects of nitric acid, is used to isolate the metal from technical residues. Gold-containing scrap is boiled in acid, then washed, dried and melted. Using this method, you can get from 10 g of scrap of 585% purity to 6.5 g of gold of 900% purity.
- Refining of precious metal from gold chloride solution. Occurs by reduction with iron sulfate. The disadvantage of this method is the introduction of another base metal impurity into the technological process, which increases the consumption of chemical reagents and the amount of by-products.
- Processing of gold-containing grinding powders. The feedstock is immersed in special reactors for dissolving gold, where a mixture of nitric and hydrochloric acids is added in proportions of 1:5 and heated to +90...+100°C. Then the solution is filtered and gold is precipitated with hydrazine hydrochloride, adding copper powder. After which the refined metal is dried, melted and sent for further purification, and the copper slag is shipped to a copper smelter.
- Isolation of precious metal from photographic remains. It is produced by adding Na2CO3, mixing with an alcohol solution of aniline, followed by exposure to light. In this case, the gold completely precipitates.
- Processing of products made from gold-plated non-ferrous metals and alloys.
- Method of gold precipitation using hydrazine hydrochloride.
- Electrolytic refining method.
Gold refining is possible wherever there are raw materials for it. And the starting raw materials can be memory cards of old equipment, parts of radio tubes, buttons, phone batteries, transistors and much more. Let's consider some options for isolating precious metal from used household appliances and several methods that allow you to refining gold using the artisanal method in home laboratories.
Refining at home
To refine gold at home, you will need special reagents and workspace arrangement. More often, electrolysis is used at home, i.e. gold refining from radio components and motherboards. First you need to prepare an anodic solution and acids. Then heat the sulfuric and hydrochloric acid to +25°C, while conducting a current of 0.1-1 A/dm². The cathode is lead or iron. In order for the metal to completely dissolve, the precipitate must react to the current. The absence of current indicates that the process was successful.
When refining gold using copper sulfate, the same steps are performed as described above. But cyanide solutions, alcohol and iron filings are also present. Since current and iron are incompatible, chloride solutions are taken instead of the latter. An auxiliary reaction is carried out to detect gold in a metal composition.
A scrap is placed in a flask with hydrochloric acid and heated. At the same time, HNO is gradually added until everything is completely dissolved. After this, the liquid cools and settles. Filtration is carried out: water is added, the precipitate is separated from the liquid. Gold filtrate is found in the sludge.
To extract gold at home, you need the following equipment:
- clean crucible;
- tweezers, which are used to transfer zinc into molten gold;
- titanium or steel stick/knitting needle for stirring the solution;
- flask for solutions made of fireproof glass;
- electric stove with a closed spiral for electrochemical refining;
- burner.
Extracting gold from memory cards
Gold can be obtained from the gold-plated contacts of computer memory cards using hydrochloric acid and hydrogen peroxide, and the same solution can be used several times.
It should be remembered that when performing any actions with chemical reagents, it is imperative to use personal protective equipment and follow safety precautions: carry out all experiments either in the open air or in a fume hood.
First, prepare the source material by cutting off all the gold-plated contacts from the cards with special scissors. Then 500 g of scraps are weighed for etching.
The solution is prepared from a ratio of 4 to 1, that is, take 1 part hydrogen peroxide to 4 parts hydrochloric acid. When peroxide is added to hydrochloric acid, the first decomposes with the release of atomic oxygen. And it, in turn, becomes a catalyst for the reaction of copper with hydrochloric acid and the formation of copper chloride. To avoid partial dissolution of gold, you need to monitor the temperature in the room: it is advisable that it should not be higher than +20...+25°C.
Pour the solution into the cuttings placed in a glass container. 15 minutes after the start of the reaction, the first gold begins to float. After 26 hours, it is separated from the sludge by 90%, and only copper remains on the scrap. All the gilding settles to the bottom of the jar; after a couple of days, the solution can be poured into another container, and then passed through a coffee filter.
The refined gold from the filter is dissolved in aqua regia: to do this, add 60 ml of 36% hydrochloric acid and 3 ml of 65% nitric acid into a gold-plated glass. A violent reaction occurs, as a result of which after 10 minutes all the gold plating dissolves. Now you need to precipitate the gold from the solution. To do this, take sodium pyrosulfite, dilute it with distilled water and pour it into a glass with a metal solution, stirring. In this way, refined gold is deposited at the bottom of the container with the solution. The essence of deposition with pyrosulfite is the release of sulfur dioxide, which, in turn, reduces the metal. Therefore, the reaction occurs with a slight delay.
Now the resulting intermediate product needs to be melted, for which the raw material is placed in a crucible and burned with a burner. To melt metal at home, acetylene, gas welding or propane torches are used. A propane torch does not require an acetylene generator and carbide, therefore it is the most convenient. The resulting ingot of precious metal is weighed on scales: from 500 g of memory card waste, 2.91 g of refined gold can be obtained.
The impact of precious metal mining on the state of the global economy
Fluctuations in the price of gold on the world market are influenced by the relationship between the demand for the precious material from the main branches of industrial production and the supply of companies specializing in mining. Buying shares of companies is a common method of investing capital, despite the unpredictability of annual metal production.
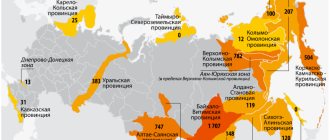
Until recently, South Africa retained the first place in the world in the production of the precious metal, but now China remains the leader, despite the high costs associated with mining and the dangerous working conditions of miners.
In North America, mining production of precious metals has decreased by almost a quarter compared to previous periods. Low content of valuable components in ore requires large physical and financial costs.
Gold mining is a labor-intensive and financially expensive process.
Even in less stable gold mining areas there is no trend towards an increase in production growth rates. Companies specializing in gold mining are sometimes faced with the issue of the inexpediency of developing new deposits.
As a way out of this situation, the following solutions are being taken:
- new technologies are being developed to extract the precious component from primary production waste;
- The rate of production of known deposits is increasing.
Global gold production peaked in 2015. The lion's share of the increase in the indicator occurred in Mexico. World gold production affects the cost per ounce on the foreign exchange market, and the dynamics of rising world prices has a positive effect on the profitability of production.
The leading countries in gold mining in the world are geographically located on different continents. The reserves of the precious component in the depths of the states determine the leading role in the mining market.
Among the countries of the world, the championship belongs to China, followed by Australia, Russia, the USA, and Canada.
Extracting gold from microcircuits
Refining gold from microcircuits is a somewhat more labor-intensive process, since the amount of material unnecessary for the final result in the raw materials (controllers, processors) is much higher here, and it is therefore more difficult to isolate the precious metal.
First you need to get rid of the legs of the microcircuits, since they consist mainly of brass and iron: they are removed with a knife or scissors, leaving only those plated with gold. This is necessary in order to save acid, since reagents are quite expensive today.
Then you need to burn the prepared raw material in an oven, let it cool and crush it with a plastic pestle or rod in a suitable container, possibly metal, given that everything will be covered with ash. The crushed raw material is transferred to a flask, where pure nitric acid is poured to dissolve copper and other metals. Approximately 85% of impurities are removed in this way. The resulting blue solution is filtered. The filtrate containing silver is treated with a concentrated salt solution. As a result, silver chloride precipitates in the form of white flakes, similar to cottage cheese.
The material remaining in the flask must be treated with aqua regia, and this process is best carried out under a hood or through a gas outlet pipe. The resulting green solution containing gold is also filtered and then treated with either sodium pyrosulfite or ferrous sulfate. The powder can be poured directly into the container with the solution, stirring with a glass rod, and then left for several hours so that all the gold precipitates.
Next, the product needs to be filtered, the filter dried and proceeded to firing. Melting of the metal is carried out in a crucible in a muffle furnace. For firing you will need a screwdriver, tweezers, tongs to remove the crucible from the oven, and a metal rod to stir the product. It is also good to prepare a little nitric acid in advance in the flask where the resulting calcination product will be transferred. This is done so that the acid cleans the gold and turns it yellow.
The unnecessary edges of the filter are cut off and placed in a crucible, squeezing and compacting. After this, the crucible is placed in a muffle furnace for 12 - 15 minutes. The resulting product, while still hot, is placed in nitric acid, placed on a stove and boiled for some time until the gold bar is completely cleansed.
There is also refining using tin chloride, pickling using ferric chloride, metal purification using zinc and many other chemical methods.
Catalogs of radio components and passports for equipment as sources of information about the content of valuable metals
You can find out whether there is gold in radio components by studying the technical data sheet. The directory, among other information, contains the composition and release date of the product. Now such catalogs are available for free download, most often they are presented in the form of tables.
Most often, the precious metal can be found inside Soviet radio components. It was used to cover the pins of microcircuits, connectors, grids of radio tubes, and substrates for conductors inside transistors.
Among modern technology, solar metal is less common; it is usually replaced by tungsten.
As for USSR technology, the precious metal content inside microcircuits can reach 5%. Most of the gold metal comes from old army equipment; the Soviet Union never saved on this industry.
Legal aspects
Thus, having certain knowledge and a set of available tools, it is possible to refining gold from old household, office and even military equipment not only in laboratories, but also at home.
It should be borne in mind that the law on the illegal circulation of precious metals is still in effect, which covers every illegal production of gold, no matter what kind of trash and waste it was mined from.
Therefore, it is wiser not to violate the law, which provides for violation of paragraph 191 of the Criminal Code of the Russian Federation with forced labor for up to 5 years, or imprisonment for the same period with a fine of up to 500,000 rubles.
List of radio equipment and gold-containing elements installed before 1985-1986
Soviet electronics produced before 1985-1986 deserve special attention. Such devices contain the maximum amount of precious metal, so there is no need to rush to part with old equipment from the USSR era.
Many devices, such as televisions, tape recorders, radios, contain gold “filling”.
RB-3 Radio components
The list of gold-containing parts of radio equipment will be as follows:
- Transistors. A large content of precious metal is found in the KT101, KT103, KT117, KT603, KT613 series, its main concentration is under the crystal or on the legs.
- Connectors. They are covered with the thinnest layers of solar metal.
- Radio tubes. The models GMI-11, 12P17L, 6V1P are especially different. Radio tubes, in addition to gold, may contain platinum, silver, tantalum, and palladium.
- Semiconductors. A little precious metal is contained in diodes, light-emitting diodes, and zener diodes.
- Capacitors. They contain the largest volume of solar metal.
- Wrist watch. The body of the product was sometimes covered with gold.
- Computer parts. Gold-containing parts can be part of processors, connectors, etc.
The older the Soviet product, the more valuable materials may be inside. The metal used in radio components is 999 fine. The thickness of gold surfacing on radio components is extremely small and is measured in microns.
What hazards do chemical reactions and processes entail?
Naturally, you need to understand that any work with acids can have serious consequences.
For example, working with aqua regia, or rather the refining process where metals are dissolved, takes about six hours.
In this case, a strong release of nitric oxide occurs.
Therefore, in no case should such work be carried out in closed lighting; the windows must be open so that fresh air has a constant opportunity to enter the room.
One breath of poisoned air with oxides can cause a sudden and painful death, since doctors simply do not have time to provide first aid.
Necessary tools and substances
To extract precious metals, you do not need special equipment. All the necessary equipment is available for free sale, and many accessories can be found in your home workshop.
List of necessary reagents and tools for refining by chemical method:
- laboratory measuring cups made of durable glass with a volume of 1000 ml (2 pcs.) and 150 ml (1 pc.);
- melting utensils with a heat resistance level of over 500°C;
- thick rubber gloves;
- rubber apron;
- protective glasses;
- respirator;
- glass or ceramic stirring rod;
- chemical reagents necessary for the chosen method;
- funnel and filter paper;
- burner.