Let us pay attention to a natural and quite effective sorbent that helps improve the quality of liquid used for food purposes. Let's take a closer look at zeolite: what it is, application, characteristics, useful properties, features - we'll go through all the important points of its use so that you understand how relevant it is.
Looking ahead a little, we note that today they have learned to synthesize it by growing crystals of the desired, that is, geometrically correct shape - with a structure in the form of a tetrahedron and cavities of the same size, filled with H2O molecules and alkaline earth metal cations. Considering its enormous relevance, artificial production has become a real find and has made it possible to begin comprehensive development of a wide group of drugs and all kinds of cleansing agents.
Natural zeolite: what is it?
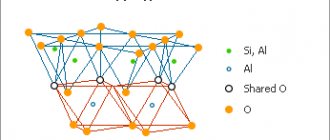
This is a mineral, or, more precisely, a group of them - framework aluminosilicates, the origin of which is sedimentary-volcanic. In essence, it is a hardened mineral-lava mixture that crystallized after entering salt water, sea or ocean.
If you make up its external description, then in the form of an agglomerate it looks like very dense clay, but white or colorless, with a pearlescent or glassy sheen. Less commonly, it is brownish-red, with green-blue splashes.
Its chemical composition is as follows:
- silicon – 75%;
- aluminum – 11-15%;
- water – 9-10%;
- other elements – 1-2%.
It is the latter, despite their small share, that determine the type, and therefore the name, of the mineral. Thanks to them, heulandite, erionite, phillipsite, lomonite, ferrierite and, most important for modern science, mordenite and clinoptilolite are distinguished.
Let's talk separately about synthesized zeolite: today about 150 of its varieties can be obtained artificially. The difference between them is the pore size and components. Each of them has a base metal, which is written first, and a lattice of a certain structure (the most relevant are A and X). That's why you often see names like NaX or CaA.
There are also high-silica aluminosilicates, which are considered especially valuable due to completely unique catalytic and adsorption properties. The best and most expensive type is ZSM.
Varieties
Depending on their origin, zeolites are:
- natural;
- artificial.
Depending on the structure of the structure, minerals are distinguished:
- isometric (most common);
- fibrous;
- lamellar.
Depending on the chemical composition, up to 50 varieties of zeolite are distinguished. The most common of them:
- mordenitis;
- natrolite;
- ferrierite;
- heulandite;
- laumontite;
- scolecite;
- clinoptilolite;
- erionite;
- phyllylaite.
How the stone was discovered
Back in the 18th century, it was discovered by Axel Kronstedt: the Swedish chemist conducted experiments on heating stilbite and noticed that the surface of the prototypes began to swell and bubble. Having become interested, the scientist empirically found other aluminosilicates with similar properties. Their family received the name “zeolites”, composed of two ancient Greek words: “boil” - “zeo” and “stone” - “lithos”.
After which, for more than 150 years, the find had exclusively theoretical value, because the researchers did not know how to properly use it in practice. The situation changed at the beginning of the 20th century, when, due to the high absorption capacity of the stone, they began to remove heavy metals, oils and nitrates from petroleum products. Later, the sorption effect was used for the purification of drinking liquids.
A new round of popularity was provided by such a sad event as the accident at the Chernobyl nuclear power plant. Scientists under the leadership of Vasily Ivanovich Bogatov developed a simple but effective means of combating radiation sickness: they mixed zeolite powder with water and gave liquidators this solution. Later, based on this “chatterbox,” the drug “Litovit” was made, which perfectly removed radioactive strontium and cesium from the body.
In parallel with this, sokirnite was used in the Dnieper and Pripyat basins to disinfect wastewater, and was also introduced into plant and vegetable growing - they “fed” growing seeds with it in order to improve their germination. Experiments even began in the field of cosmetology - the sorbent was useful for extracting harmful substances from the skin of the face and body, and they began to make masks that were safe for human health. And, of course, filtration of the flow taken from the central water supply system is a classic use of these aluminosilicates.
Price
Zeolite has a wide price range. The cheapest are artificial samples used in industry. Their cost starts from $0.6 per 1 kg. Unprocessed stones of natural origin can be purchased for $9-10 per 1 kg. A piece of jewelry with this mineral, framed in a jewelry alloy, will cost approximately the same.
Natural collection quality zeolites cost between $9-11 per copy. The price of unusual large specimens starts from $20 for 1 piece and above.
What is good about zeolite: properties of the mineral
Due to its unique frame structure, loose but strong, and its special chemical composition, it has the following characteristics, useful and important:
MBFT-75 Membrane for 75GPD
SF-mix Clack up to 0.8 m3/h
SF-mix Runxin up to 0.8 m3/h
- Impressive adsorption - its crystal lattice reliably binds a variety of negative substances, from radioactive and heavy metals to nitrates and toxins.
- Active ion exchange - it absorbs particles of the medium (in our case, H2O) and releases minerals to it due to its porosity.
- Catalysis – it significantly accelerates chemical reactions, which is especially important in oil refining.
- The molecular sieve effect - the dimensions of the internal planes and even the pores of different types of stones are strictly individual. Having selected one or another of them, you can sift out or absorb only the necessary substances, for example, exclusively strontium or cesium, and pass the remaining components further through the filter.
Useful properties for treatment
Various types of zeolites have long been successfully used in several branches of medicine. For example, it is used to make products that help with gastrointestinal disorders and food poisoning. In cosmetology, anti-aging masks, cellulite scrubs, and therapeutic muds are made on its basis.
In general, he:
- activates blood circulation;
- normalizes the functioning of the gastrointestinal tract;
- strengthens the muscles of the heart and blood vessels, helping to get rid of tachycardia, ischemia, and bradycardia;
- removes poisons from the body, and also reduces the negative impact that harmful substances could already cause.
Healthy people can also take courses of taking medications containing zeolite - for prevention, because with systematic use of the mineral:
- has a beneficial effect on the nervous system;
- increases immunity;
- normalizes acidity;
- removes toxins and nitrates from food, drink, and air;
- maintains the balance of microelements, which helps prevent a number of diseases.
This results in a surge of energy, strength and vigor, and an improvement in well-being.
Taste properties of water after zeolite
In addition to heavy metals and harmful radionuclides, the stone helps remove fungi, bacteria, and organic contaminants from H2O, and therefore normalizes its organoleptic properties. After such treatment, the unpleasant odor goes away, the sourness and bitterness disappear, perhaps the alkaline level increases slightly, but drinking such a liquid or cooking something with its use becomes much more pleasant, and most importantly, safer for health.
Therapeutic effect
Zeolite has a beneficial effect on the body. It has antioxidant properties, which has a positive effect on the immune system. The mineral removes heavy metals such as lead, cadmium and mercury from the body, and relieves intoxication caused by alcohol and drug poisoning.
People suffering from diabetes are recommended to use zeolite to speed up wound healing. Masks, scrubs and mud baths with this mineral help rejuvenate the skin and eliminate cellulite.
Zeolite is useful for problems with the digestive tract. It normalizes stomach acidity and helps with bloating and diarrhea.
This mineral is also used in the fight against cancer. It helps reduce the side effects of chemotherapy and radiation.
Mining locations
Aluminosilicates are found all over the planet, but they are found in the largest quantities in the Urals. If we talk about Russia, then the Kemerovo region is also rich in them, and there are also a lot of them on the lands of the Krasnoyarsk Territory.
If we talk about the world as a whole, then countries where volcanic activity was observed, that is, Japan and Iceland, and a number of African states have impressive reserves of “boiling stones”.
It is interesting that the rock, as a rule, does not lie very deep, and several types of stone can be found in the same vein. This is due to the fact that the lava mixture that solidified and formed them was heterogeneous in its chemical composition.
A discovery that was considered useless for 200 years
In 1756, the Swedish academician A.F. Konstedt, who devoted his entire life to mineralogy, witnessed an interesting phenomenon. When the stilbite was heated, small bubbles formed on its surface, as if it began to boil. As it was later found out, this reaction was accompanied by the release of H2O. Several minerals that give such a reaction have been identified, all of them are called Zeolite. Unfortunately, at that time no use was found for “boiling” stones. The discovery remained virtually unnoticed. They remembered him only two centuries later. It turned out that zeolites can serve as excellent filters for purifying water from salts. This inexpensive mineral began to be used in water purification for industrial needs.
Where is natural zeolite used: application of the mineral
It is more than relevant and in demand not only in its natural form, but also in its synthesized form - in several key areas of our time, and we will consider each of them right now.
Industry
Aluminosilicates are actively used in the following areas:
SF-mix manual up to 0.8 m3/h
AMETHYST - 02 M up to 2 cubic meters/day.
Aeration unit AS-1054 VO-90
- Construction - added to concrete mixtures to strengthen them, as well as to speed up their hardening.
- Oil production and processing of the resulting products - crushed stone acts as an additive that removes sulfur components and prevents dehydration and desalting. It is also considered an effective catalyst for distillation.
- In the chemical industry, they are included in mineral fertilizers, increase the shelf life of raw materials, prevent caking of bulk materials, and strengthen granular substances. They are also an environmentally friendly alternative to phosphates in detergents, in particular in washing powders.
- Housing and communal services - with their help they purify waste and drinking water, simultaneously making it softer.
Synthetic zeolites deserve special attention: the use of their technical varieties in industry is especially important in the production of rubber, plastic, leather, glued products, containers, and packaging.
Agriculture
In crop production, it is in demand for several qualities:
- as an enricher - manganese, magnesium, cobalt, iron and other microelements;
- as a hardener that prevents destruction and extends the shelf life of fertilizers;
- as a growth accelerator for agricultural crops, increasing productivity, preventing the absorption of harmful substances from the soil, and protecting against diseases.
In livestock farming, crushed stone is an important component of feed and gives it an important boost of minerals, and also prevents it from becoming moldy. Plus, it promotes better absorption of nutritional components, due to which the same cattle reproduce more actively and gain useful weight faster.
The sorption properties of aluminosilicates are used in the manufacture of bedding, on which animals and birds subsequently sleep; finished products absorb odors and moisture more effectively.
Preparations based on “boiling” stones are also poured into man-made reservoirs so that these products destroy viruses, bacteria, and foreign microorganisms in ponds and lakes, thereby creating a favorable environment for fish breeding.
Environmental protection
The natural mineral zeolite will help maintain an acceptable environmental situation (and minimize the harmful effect) in the event of the following dangerous situations:
- large-scale spill of oil, fuel oil and similar chemicals;
- release of ammonia, methane, carbon dioxide and other gases;
- contamination of soil and liquid pools with radionuclides and/or heavy metals;
- ingress of toxins into reservoirs, wells, wells.
Solving home or household problems
Different types of stone are used in the following cases:
- purification and softening of H2O supplied through pipelines of residential buildings and offices - crushed aluminosilicates are a component of the filter backfill;
- saturating the soil with oxygen to accelerate the growth of flowers and plants planted in it;
- absorption of smoke and tobacco smell, stone chips also effectively neutralize unpleasant stagnant air or persistent kitchen aromas;
- absorption of moisture in mixtures and cat litter;
- preventing mold in the bathroom, refrigerator, closet and other places where vegetables are stored - a small fabric bag filled with crushed sorbent is enough for this;
- cleaning of aquariums - slowing down flowering, absorbing waste from fish, crustaceans, mollusks, algae;
- drying and refreshing shoes - inside the shoe, sneaker or boot you need to put a bag of loose mineral.
Main characteristics
The color palette of the nondescript mineral is extremely scarce and lies between translucent white and pale pink. Beige, dirty gray, milky shades - all of them can color a useful gem. The intensity and nature of the color depend on the chemical impurities in the chemical formula of the stone. The general formula is presented in the form of aluminosilicates, water molecules and chemical impurities in the form of alkalis and oxides.
Interesting! The mineral was discovered in 1756. However, at that time this discovery was not appreciated and the new gem with “explosive” properties was soon forgotten. The oblivion lasted about 200 years, and ended at the moment when the question of new ways to purify drinking water became acute. Then scientists found out that zeolite is an excellent adsorbent stone and purifies water without changing the taste. From now on, the era of the inconspicuous mineral begins.
The chemical composition of zeolite is as follows: the base is SiO2 silicate, to which is added aluminum oxide, a cation with a positive valency (potassium, calcium, sodium, barium, etc.) and water molecules. Thus, the purifying function of zeolite is determined by the binding properties of these same cations, which, like a sponge, “absorb” the impurities contained in drinking water.
The zeolite mineral is quite fragile and easily susceptible to mechanical stress. According to the general measuring scale of mineral hardness (Mohs scale), the mineral has 4-5 units, which characterizes it as a fragile gem. The density is low (less than 2 g per cm3), but zeolites with barium cations have an increased density (up to 2.7 g per cm3). Presented in the form of a crystalline “brush”, it has a glossy shine and a translucent or transparent structure. The color palette, as mentioned above, is diverse in a range of pale shades of colors.
How zeolite was used for water in history
Having analyzed the information already available, we will highlight the main milestones:
Main table dispenser AquaPro 919H/RO (hot and cold water)
Main table dispenser AquaPro 929CH/RO (cooling/heating)
Floor dispenser AquaPro 311 (empty, without cooling)
- After the Chernobyl accident, it was bred and drunk to reduce damage from radiation sickness.
- In the 90s of the twentieth century, wastewater began to flow through its two-meter embankment for disinfection.
- In the 2000s, having already assessed its effect, it was used as a filter media that eliminates compounds of hydrogen sulfide, methane, and ammonia, but retains proteins, vitamins, amino acids, and harmless and useful organic matter with a complex structure.
Well, since the 18th century it has been a real find for traditional medicine. “Boiling stones” were immersed in the liquid, they settled it, believing that it became healing, and in some ways they were right, because drinking it strengthened the body.
Pyramids
Pyramids are used to neutralize geopathogenic zones. These can be natural sources, for example, geological faults or dry river beds, or they can be man-made - power lines and other sources of electromagnetic radiation. If a person finds himself in such a zone, his condition worsens sharply: insomnia, tension, and pain appear. Zeolite relieves these symptoms by taking on bad energy. In everyday life, the pyramid is placed next to electrical appliances: computer, TV, refrigerator. Once a month, the pyramid must be cleaned - fry it in the sun, keep it in the moonlight, or wash it in running water. This will remove the negative charge. Symptoms of energy tension can be relieved by direct contact with the pyramid. For headaches, it is applied to the forehead, for heart disease, it is applied to the chest, for back pain, the pyramid is heated and the sore spot is lightly massaged, for convulsions, an injection is given with the top of the pyramid. For skin diseases and varicose veins, zeolite baths are used.
Zeolite for water purification today
Nowadays it is artificial stones that are mainly used - they are included in installations as electron acceptors and donors. Due to their crystalline structure and “spongy” absorption effect, they:
- Heavy metals, radioactive elements, phenols, viruses, nitrates, ammonia, ammonium, pesticides, and organic contaminants are removed from the liquid.
- They reduce the concentration of chloride and fluoride ions, remove hardness salts, and promote softening.
Naturally, aluminosilicate of a specific type, for example, clinoptilolite, cannot remove all of the above, because its cells have a certain geometry, which means they retain only particles of the appropriate size. Therefore, one download “from everything” is, unfortunately, just a trick of marketers.
Contraindications and side effects
During the first week of taking the drug, you may experience dizziness and a slight feeling of nausea. These effects are considered a normal response of the body to detoxification.
Zeolite has a number of contraindications for use:
- pregnancy;
- lactation period;
- children under 18 years of age;
- individual intolerance;
- gastric and duodenal ulcers in the acute stage.
Zeolite is an effective natural remedy for cleansing the body of toxins, fermentation and decay processes. If you have any questions or have results on using the mineral as a detoxifier, write about it in the comments below.
Mineral based water filter
What is zeolite used for - natural or synthetic? It will be a more practical filler for a purification plant than, say, the popular quartz sand. Because it gives results relatively faster and at the same time requires less liquid for rinsing.
It is also suitable for effectively removing unwanted contaminants from both flow and standing H2O. When poured in the form of granules or powder, it completely restores natural organoleptic characteristics, that is, normal smell and taste, removing bitterness and/or unpleasant “sulfur” aroma.
Statistics speak better than words; Thus, a catcher loaded with correctly selected stone chips can reduce the level:
- chlorine – up to 65%;
- aluminum – up to 44%;
- copper – up to 78%;
- phenol – up to 83%;
- iron – up to 85%;
- lead – up to 94%.
Artificial acquisition
The industrial synthesis of zeolites is carried out at relatively low temperatures (up to 200 °C) and saturated water vapor pressure in an alkaline environment (pH 14) from a highly reactive material. The properties of natural zeolites are changed by treating them with various acids, alkalis and salts. Thus, acid treatment of clinoptilolite can lead to its dealumination without destruction of the frame, accompanied by an expansion of the entrance windows, thereby increasing adsorption to water and benzene vapor.